Coronavirus / COVID
Latest News
Latest Videos

CME Content
More News

Today serves as a reminder to everyone that there are still no diagnostic tests or FDA-approved treatments for the post-viral condition.
Insights from Jacob Teitelbaum MD on Long COVID Awareness Day.

Saskia Popescu PhD, MPH, MA, FAPIC on understanding the emergence of the origin for future pandemic preparedness, promoting global health security, and addressing long-term health impacts.

A new study shows how correcting misinformation in a certain way can change patients’ attitudes towards vaccination.

A new study examined the association of COVID-19 infection with outpatient care utilization.

A reduced risk of individual symptoms associated with the condition was reported when patients were administered the therapy within 2 days of admittance.

A new study offers some clues into the pathophysiology and biomarkers of post-acute Sequelae of SARS-CoV-2 (PASC).

With today's vote, the federal agency’s Advisory Committee on Immunization Practices (ACIP) is now recommending people 65 years of age and older get 1 more dose of the 2023-2024 formula of the COVID-19 vaccine.

Shionogi’s investigational therapy, ensitrelvir, demonstrated a median time-to-symptom resolution of approximately 1 day shorter in the antiviral group versus placebo.

Participants of a recent study who experienced more daily cognitive symptoms had a greater likelihood of reporting moderate interference with functioning, less likelihood of full-time employment, and greater severity of depressive symptoms.
CDC reveals trends in M pneumoniae infections before, during, and after the pandemic.
Study compares immune responses in cancer vs. non-cancer patients.

The Centers for Disease Control and Prevention (CDC) considers changing its previous guidance.
Increased risk of post-acute conditions (PASC) in SARS-CoV-2 positive patients.
The self-amplifying mRNA vaccine, ARCT-154, had an extended response compared to the BNT162b2 vaccine and was approved in Japan back in November.

CDC endorses the role of antiviral drugs in combating COVID-19.

This news comes after the World Health Organization (WHO) recently granted the vaccine an emergency use listing (EUL), and continues to become available to more countries.
The National Foundation for Infectious Diseases (NFID) medical director weighs in on this year’s respiratory virus season and offers some strategies to prevent transmission.
A small study offers some more information as to potential biomarkers for the ailment and may provide a better understanding of its path to potential treatments.
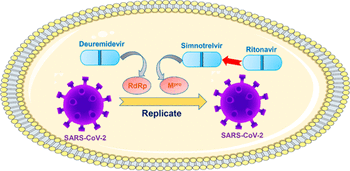
Early administration of simnotrelvir plus ritonavir shortened the time to sustained resolution of COVID-19 symptoms.

Investigators uncover a convergent evolution of enhanced innate immune antagonist expression associated.

Here are some insights into the comparative analysis of COVID and non-COVID pneumonia.

Myopathy, with metabolic disturbances and amyloid deposits, is discovered in persons with Long COVID who experience post-exertional malaise.

Results of a study by Yan Xie and colleagues highlight the severe impact of COVID-19, with higher death rates, increased risks of long-term complications, and greater infectivity compared to seasonal influenza.

A study used real-world CDC data to calculate the number of people who needed to be vaccinated to prevent both medical events in the greater community.