
Declaration allows mobilization of funding, resources to address the virus.
A new study finds there are more pharmacies than physician practices in these areas; however, these inequities offer an opportunity for pharmacists to step-in and provide vaccine education and administration.

This study looks to identify safety, efficacy of the vaccine in the youngest pediatric populations.
The benefits outweigh the possible rebound of symptoms, according to an investigator who studied the antiviral.
The modified spike protein in this next-generation bivalent candidate is part of the companies' strategy to try and provide vaccines with longer and more durable protection against the virus.

Finding that the COVID-19 pandemic disrupted best antibiotic practices, the CDC announced further efforts to curb treatment-resistant infections.
The aim is to increase vaccination rates and awareness in the state with the second most confirmed cases.

This regulatory review is for the prevention of recurrent vaginal yeast infections. And if approved, it would be a second indication for the antifungal related to these infections.

At AIDS 2022, Gilead presented data suggesting Biktarvy was a safe and effective complete HIV treatment regimen, including for those coinfected with hepatitis B.
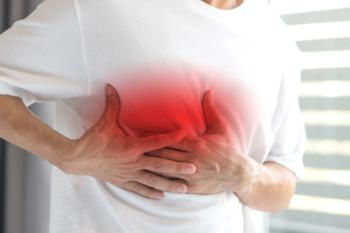
The highest rate of myocarditis or pericarditis after mRNA COVID-19 vaccination occurred among young men ages 18 to 24 after receiving a second dose of the Moderna vaccine.
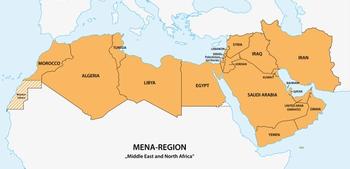
HIV epidemics are emerging among key populations in the Middle East and North Africa, and the region ranks lowest globally in response indicators.

The model suggests screening unvaccinated students could reduce absences by 80% compared to reactively closing classes with infected students.

With antimicrobial resistance a rising threat, the World Health Organization is working to heighten awareness of the urgent need for new therapies.

Clinicians offer insights on a variety of elements surrounding boosters.

What steps need taken to end viral hepatitis by 2030? “It all comes down to testing,” said Carl Schmid, executive director of the HIV+Hepatitis Policy Institute.

Implicating adenovirus for hepatitis of unknown cause in children is confounded by its absence in some cases, and from all tested hepatic tissue.

Viral hepatitis “can and should be managed within primary care,” says Dr. Thomas Robertson.
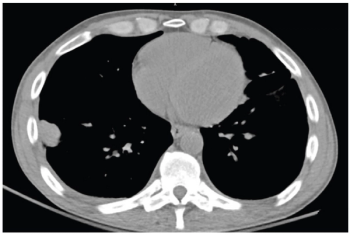
A patient presented with a history of uncontrolled HIV and a history of polysubstance use.

An HIV-positive woman in her 60s has recovered from a simultaneous heart and kidney transplant.
Here is a review of the literature for this class of antibiotics.
Investigators designed a quasi-experimental pre- and post-intervention analysis comparing a pre-intervention cohort receiving IV drip antibiotic infusion with a post-intervention cohort receiving IVP administration.
Frequent recurrence of Clostridioides difficile infection drives up financial burdens of the disease and underscores the need to improve access to treatment, infection prevention, and new therapies.

Last weekend, the World Health Organization declared the monkeypox viral outbreaks a Public Health Emergency of the International Concern (PHEIC).

A new study demonstrated that gut organisms can alter drug availability by biotransformation on a significantly broader scale than previously expected.


As the pandemic enters its third year, fatigue and frustration grow, but there is hope for the future.
Working to Fight AMR is working in Washington DC to help pass policies in Congress to help alleviate this significant issue.

Although this form of prophylaxis is highly protective, there are situations in which breakthrough infections occur.

A robust stewardship program requires a comprehensive investment in technology and staff.