The fast-moving Omicron variant demonstrates a high transmissibility, but not as significant a hospitalization rate or mortality.

The fast-moving Omicron variant demonstrates a high transmissibility, but not as significant a hospitalization rate or mortality.

COVID-19 patients who underwent prior weight-loss surgery were significantly less likely to experience severe disease outcomes.
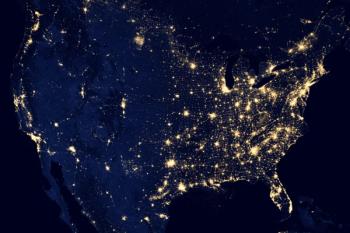
The US is on track to reach new records for 7-day average cases, though death rates remain lower.
A phase 3 trial shows one dose is 91.7 % effective against severe disease.

The oral antiviral therapy proceeds the first indication for the drug class by just a day; the US will now have multiple pills to treat COVID-19.
In a study of more than 600 elderly Japanese, being overweight was a protective factor against Clostridioides difficile infection (CDI).

Risk of inaccuracy with pulse oximeters and clinician knowledge gaps in people of color may affect sepsis evaluations and impact treatment plans.

Stigma, and a lack of communication and awareness all inhibit HIV PrEP uptake, but digital health and new forms of PrEP are aiming to increase users.

This is the first pill to treat COVID-19 in trying to prevent progression to severe disease and possible hospitalization.

COVID-19 patients were not at increased risk of Clostridium difficile infection. However, those already infected with C diff were more likely to experience severe outcomes.
A study of mRNA vaccination in older people and people with comorbidities found vaccine efficacy of 69% against COVID-19 infection and 86% against death.
This novel therapeutic option represents the first long-acting injectable approved for maintenance of HIV-1 suppression.

The administration is going to buy 500 million at-home rapid COVID-19 tests, and make them available to Americans. They also plan to use federal resources to provide pop-up clinics and get more pharmacists involved in vaccinations.

Here’s where you should sit on the bus to avoid COVID-19 droplets.

Incidence of hepatitis B has fallen in the Asia and Pacific region, but progress against hepatitis C in the region has lagged and morbidity and mortality continued to rise, underscoring a need to improve diagnosis and linkage of care, according to a recent report.
Less than 3 weeks after it was first reported, Omicron becomes the dominant COVID-19 variant in the US.

In the age of COVID-19, providers caring for patients with emerging diseases do not rely entirely on clinical guidelines, but also consult online resources that are updated more frequently. This needed integration helps providers adapt to the best-available evidence into bedside care when guidance is lacking.
Epidemiologic and clinical lessons for the uninitiated.
Respiratory syncytial virus (RSV) cases decreased due to COVID-19 precautions. Investigators estimated the rate and timing of RSV resurgence over the next few years.

Nearly 20% of infants were overweight or obese by 1 year of age regardless of HIV exposure, a new study in South Africa found.

Two different doses of the company's mRNA vaccine were found to increase antibodies by up to 83-fold.
Will this winter bring the feared twindemic of SARS-CoV-2 and influenza, or will another common infection reemerge?
Planning travel and social gatherings as Omicron gathers steam requires extra preparation and a degree of flexibility.
A study of patients hospitalized with COVID-19 pneumonia found namilumab, but not infliximab, significantly reduced inflammation and CRP concentration.
Pfizer-BioNTech will be testing 3 doses of their vaccine in children older than 6 months and younger than 5 years, instead of the original 2-dose regimen.

Children in the United States will be able to avoid potentially millions of hours of at-home quarantining under a testing program unveiled by the CDC today.
The proposed funding will include a number of measures of spending towards HIV and prevention, including community centers for PrEP education.

The CDC is recommending the Pfizer-BioNTech and Moderna COVID-19 vaccines over Janssen’s, due to 57 cases of a rare blood clotting condition.

Side effects that occur during concurrent vaccine administrations can influence future vaccine decisions.