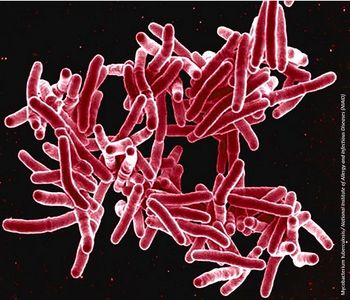
Relying solely on blood tests means clinicians may miss cases of potentially deadly M tuberculosis—and waiting days to treat suspected cases could prove fatal.

Relying solely on blood tests means clinicians may miss cases of potentially deadly M tuberculosis—and waiting days to treat suspected cases could prove fatal.

On March 25th, Contagion® hosted a live webinar featuring Helen W. Boucher, MD, Carlos del Rio, MD, Stanley Deresinski, MD, and Jason C. Gallagher, PharmD.

Health care workers involved in aerosol-generating procedures for a patient with unknown COVID-19 were protected from infection with surgical masks and standard procedures, a case review suggests.

In Singapore, it’s much easier for clinicians to order COVID-19 testing. The country also has aggressive surveillance in place. The results of those tactics are the subject of a new report.

Coronavirus cases linked to cruises have been reported to the CDC in at least 15 states.

Stay on top of coronavirus news from March 24, 2020.
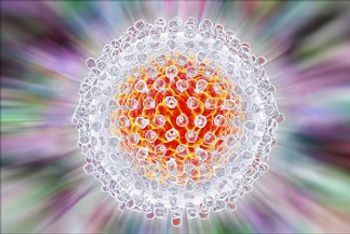
How does sharing needles impact the risk for hepatitis C among people who inject drugs?

High-dose intravenous immunoglobulin appeared to reverse deteriorating courses of COVID-19 in 3 case reports from Wuhan, China.
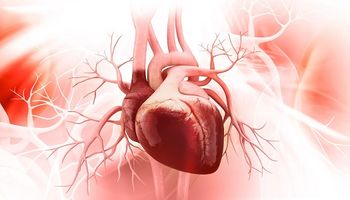
A new study is providing more information on the link between cardiac injury and mortality among patients hospitalized with COVID-19.

This is not the first infectious challenge society has faced, and nor will it be the last.

Patients facing HIV stigma may encounter difficulties with retention in care.

From epidemiology to treatment modalities, this is very much a learn-as-we-go situation.

The FDA has issued a safety alert on the potential risk of transmitting SARS-CoV-2, the virus that causes coronavirus diseases 2019 (COVID-19), through fecal microbiota transplantation.

Stay up to date with the latest COVID-19 news from Contagion.
Investigations in the US and China make a case for using convalescent plasma, a “last resort” for SARS, as an early treatment for COVID-19.

A new serological assay was developed by a microbiology lab at the Icahn School of Medicine at Mount Sinai.

World Tuberculosis Day is commemorated annually on March 24 to heighten awareness of the world’s most deadly infectious disease.

From PPE shortages to scared staff, what are hospitals currently facing and how can they respond?

The analysis included countries around the world, but the investigators also classified the data by WHO region.
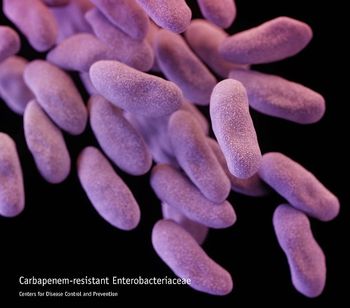
Close adherence to recommendations for preventing health-care associated transmission is key to preventing health care-associated transmission of carbapenem-resistant Enterobacteriaceae, a review of a skilled nursing facility in Arizona suggests.

Stay up to date with the latest COVID-19 news from Contagion.

Jason Pogue, PharmD, BCPS, BCIDP, shares his thoughts on a study published in the International Journal of Antimicrobial Agents.

Contagion is partnering with PER to host a live CME-Certified webinar on what clinicians need to know about COVID-19.
The impact of COVID-19 on cardiovascular health is an urgent question for clinicians around the world during this global pandemic.

A study has examined the availability of negative vaccine information online.

A Central Science report from the CAS division of the American Chemical Society details developments and recommends directions against COVID-19 pandemic.
The vast majority of patients with a documented penicillin allergy are not actually at risk from the antibiotic. New research aims to help remove the “allergic” label from some patients’ records.

Countries around the world have struggled to keep the spread of COVID-19 in check. In countries with relatively few resources and weak public health infrastructure, the challenge is even more acute.

A quick and effective response by health officials in Uganda stopped a case of imported pneumonic plague from spreading, according to a recent Morbidity and Mortality Weekly Report.

What does it look like when a health system switches from 'containment' to 'mitigation'?