
Rochelle P. Walensky, MD, MPH, signs off on the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices’ (ACIP) recommendation for individuals 6 through 17 years of age.

Rochelle P. Walensky, MD, MPH, signs off on the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices’ (ACIP) recommendation for individuals 6 through 17 years of age.
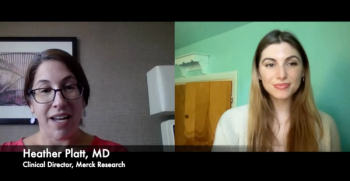
Dr. Heather Platt discusses the process of developing V116, a pneumococcal vaccine that targets the serotypes most prevalent in those 65 years and older.

The Centers for Disease Control and Prevention (CDC) is seeking more data on a possible cause of acute hepatitis in children, calling on clinicians to screen for adenovirus infections along with a broad range of possible exposures.

The federal agency says this is one of the worst outbreaks of this disease among these groups in US history, and has public health officials concerned as people gather during ongoing Pride Month activities.

This small study had 2 phases, but patients saw HIV viral suppression for about 40 weeks after receiving the combination therapy infusions.

Listen to our interview with Sanofi vaccine expert Dr. Christopher Rizzo about what this preferential recommendation means, and why high-dose flu vaccines are needed for older adults.

Patients with recurrent Clostridioides difficile infection saw improvements in fecal microbiome and bile acid after treatment with the investigational microbiota-based live biotherapeutic RBX2660.

Women who were taking hormone replacement therapy (HRT) for at least 6 months before COVID-19 infection had a 22% reduced risk of mortality.

Wearable health sensors predicted COVID-19 infection with up to 73% accuracy 2-10 days before symptom onset.

The company’s 15-valent conjugate shot becomes the first one that is approved in nearly a decade.

The largest genome sequence of S Typhi found strains of the bacteria are developing and spreading resistance to new antibiotics.

INSTIs have been thought to contribute to weight gain and metabolic syndrome, which in turn may contribute to cardiovascular events in HIV patients.

The stress of enduring an infectious disease pandemic expresses itself in a variety of mental health conditions, but the likelihood of experiencing particular symptoms depends on who you are.
Outpatients were frequently given systemic corticosteroids on the day of COVID-19 diagnosis, suggesting this usage should be further studied for safety and efficacy.
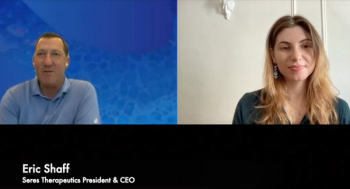
Eric Shaff, president and CEO of Seres Therapeutics, discusses the confirmatory trial findings for SER-109. Seres is filing a Biologics License Application for the recurrent C diff therapy.

Carbapenem-resistant enterobacterales in Saudi Arabia were genotyped and the course of illness characterized to help inform treatment and prevention.
Studies, advances, and authorizations continue in this paramount area within infectious disease.

The CDC director endorsed the vote, and vaccines could be available to this young pediatric population by later this week.
This past week has been extremely busy in infectious disease with a lot of vaccine news that could impact young children. In addition, GSK reported on its phase 3 study on its RSV vaccine for seniors.

Using confirmed monkeypox cases in the Netherlands, investigators estimated the average time from infection to symptom onset was 8.5 days.

Following VRBPAC recommendations earlier this week, both the Pfizer-BioNTech and Moderna COVID-19 vaccines were FDA-authorized for children as young as 6 months.

Government agencies and researchers continue to monitor the potentially troubling trend.

At separate press conferences, the CDC and WHO indicate that containing monkeypox will depend on testing, tracing, and currently available vaccines.

Out of 102 monoclonal antibodies tested, only Cv2.1169 and Cv2.3194 cross-neutralized all variants of concern, including Omicron BA.1 and BA.2 subvariants.

The South African National Department of Health has updated their HIV regimen recommendations for patients aged older than 10 years and more than 35 kg.

The FDA VRBPAC votes to recommend mRNA vaccines in youngest children; WHO moves to rename monkeypox; Anthony Fauci is diagnosed with COVID-19; and Pfizer stops recruiting for a Paxlovid trial.

Today, the FDA's Vaccines and Related Biological Products Advisory Committee (VRBPAC) unanimously voted to recommend both the Moderna and Pfizer-BioNTech COVID-19 vaccines for children as young as 6 months old.
High vaccination rates at Cornell University prevented severe disease during the Omicron surge of the COVID-19 pandemic but didn’t stop the rapid spread of the virus, even with comprehensive public health measures.

The first report from WHO Scientific Advisory Group for the Origins of Novel Pathogens (SAGO) concedes SARS-CoV-2 source remains a mystery.

Green Pharmaceuticals Inc. issued a voluntary recall of their SnoreStop NasoSpray after FDA testing found the product contaminated with the microbe Providencia rettgeri.