Community-acquired infections—including those associated with outpatient settings—are still problematic, authors say.

Community-acquired infections—including those associated with outpatient settings—are still problematic, authors say.

The international shortages of PPE have been plaguing health care workers and hospitals.

This is the first diagnostic for SARS-CoV-2 infection which uses a saliva-based collection method to be granted authorization by the FDA.

The 2 new recommendations are for HCV screening at least once for all adults aged 18 years or older and HCV screening for women during each pregnancy.

Gain more insight on clinical trials evaluating investigational agents for COVID-19.

Stay up to date on the latest coronavirus updates from April 14th.
While positive results from diagnostics can be very helpful, negative COVID-19 test results may lead to a false sense of security.

It’s too soon to know whether the novel coronavirus will be seasonal, but a study of 4 other human coronaviruses finds all 4 peak in the winter and early spring.

Free CME webinar offers a diverse look at what it’s like on the frontlines of the COVID-19 pandemic.
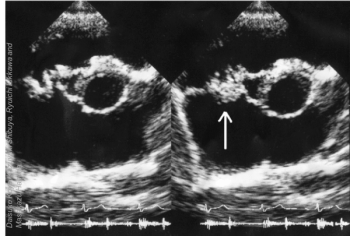
Use of prolonged IV antibiotics to treat infective endocarditis is outdated, and oral step-down therapy has been shown to be at least as effective.

Ramy Elshaboury, PharmD, BCPS, AQ ID, discusses the process of putting COVID-19 guidance in place using currently available literature and data.

This authorization could replenish the supply of 4 million N95 masks per day.

China may have been the early epicenter of COVID-19, but a new study finds Brazil’s outbreak is largely tied to travel from Italy.

This is the COVID-19 update for Monday, April 13, 2020.

The USPSTF commissioned a review on the impact of asymptomatic bacterial vaginosis screening and treatment on pregnant women with respect to preterm birth.
Thomas Dilworth, PharmD, shares tips and resources that can help busy clinicians stay engaged and up to date during the COVID-19 pandemic.

A new analysis of COVID-19 pneumonia deaths shows ICU space was insufficient, and comorbidities were a major factor.
Investigators performed avian influenza surveillance in wild bird gatherings across China between 2017 through 2019, isolating and exploring the biological characteristics of 2 different H16N3 subtype influenza viruses.

Although controlled trial results of remdesivir for COVID-19 won't be available for several weeks, early experience with compassionate use is detailed in a new NEJM report.

Loss of smell or taste has been reported to occur with COVID-19 in absence of other symptoms, heightening risk of exposure for unknowing contacts.

Emergency room doctors in Wuhan tracked their own recoveries from coronavirus using a phone app to aid researchers.
Matthew Davis, PharmD, provides an overview on the agent remdesivir.

Organizational leaders can support their health care workers fighting COVID-19 in small ways. Start by asking “what do you need?”

Here is a look at infectious disease-related FDA news from the week of April 5, 2020.

We’ve compiled a list of recalls issued by the US Food and Drug Administration (FDA) and US Department of Agriculture (USDA) from this past week:

Check out the latest coronavirus updates from Friday, April 10th.

Stay up-to-date on the latest infectious disease news by checking out our top 5 articles of the week.

The emergency use authorization will support the decontamination of 750,000 N95 respirators per day in the United States
The majority of critically ill patients admitted to the ICU in Lombardy, Italy with COVID-19 were older men and required mechanical ventilation.