
The Peggy Lillis Foundation will host its summit on Monday and has a number of speakers who will discuss topics related to this healthcare-associated infection (HAI).
John Parkinson is the assistant managing editor for Contagion. Prior to joining MJH Life Sciences in 2020, he has covered a variety of fields and markets including diabetes, oncology, ophthalmology, IT, travel, and local news. You can email him at jparkinson@mjhlifesciences.com.

The Peggy Lillis Foundation will host its summit on Monday and has a number of speakers who will discuss topics related to this healthcare-associated infection (HAI).
A key stakeholder offers his insights on the importance of the new Biden plan to eliminate hepatitis C as well as the federal strategy to get more people linkage to care for hepatitis B.

The FDA’s Antimicrobial Drugs Advisory Committee (AMDAC) voted 12-0 in favor of recommending this antibiotic and sets up a PDUFA target action date of May 29.
Since the lifting of public health restrictions, the country saw a sizable increase in incidence rates.

With an FDA approval, it would be the first new class of oral antibiotics for uncomplicated urinary tract infections (uUTI) in over 20 years.

Several years after its FDA approval, this antibiotic continues to prove its efficacy in vitro across various pathogens related to these infections.
A new study presented at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) examined a rapid cellular host response test designed to aid providers who are working in emergency departments and need quick, efficient diagnostic information.
People with COVID-19 who were hospitalized had a slightly higher rate of death than those with influenza. However, there were considerably more severe COVID-19 cases than the flu, leading to a much higher number of overall deaths associated with SARS-CoV-2.

With limited amounts of antibacterials, prescribers are presented with optimal therapy challenges as well as difficulties in trying to achieve stewardship.

The R21/Matrix-M vaccine can now be used in Ghana and could help in the prevention of a vector-borne disease that kills over 600,000 people annually.

The Veterans Administration reported a reduction in the bacterial infection in healthcare settings where infection prevention practices were continued.
The epidemiology society worked with a few medical organizations to update the guidance to limit infections.
A study looking at 5 years of data showed a decrease in the amount of cases, but with some important trends to be aware of in this population.

The US Department of Agriculture’s Food Safety and Inspection Service (FSIS) issues public health alert for salad with chicken and ham.
In patients with hepatitis D (HDV) and compensated cirrhosis, a newer antiviral therapy, bulevirtide (Hepcludex), proved to be efficacious and safe to patients.

When taken within 3 days, doxycycline given post-exposure (doxy-PEP) decreased the incidence rates of syphilis, gonorrhea, and chlamydia significantly.

The case and another probable case is being presented at next week’s European Congress of Clinical Microbiology & Infectious Diseases conference.

These maternal infections caused seizures, developmental delays, and other health issues in two newborns.
Investigators reviewed the current literature on the utility of oral vancomycin prophylaxis (OVP), and offered guidance for when this approach may be beneficial.

According to the CDC, the outbreak strain of carbapenem-resistant Pseudomonas aeruginosa with Verona integron-mediated metallo-β-lactamase and Guiana extended-spectrum-β-lactamase (VIM-GES-CRPA), has never been reported in the US prior to this outbreak.

After assessing the landscape, the company is leaving the adult RSV vaccine market.
Aaron Ferguson’s diagnosis completely changed his life’s path. Since then, he has helped others overcome their addictions and get hepatitis C (HCV) treatment. In his home state of Texas, a new health initiative is looking to get more people into the continuum of care for this curable virus.
The CDC’s recent warning of the rising incidence rates of the fungal infection is cause for concern, but understanding which patients and settings are at a higher risk is equally as important. The lead author in the CDC’s recent C auris study offers some insights for both medical institutions and the general public.

The oral RNA destabilizer, AB-161, developed by Arbutus Biopharma, started dosing its first participant for the small trial.

The virus can maintain a reservoir in these white blood cells even in those who have been virally suppressed for years.

A midsized study demonstrated no therapeutic benefit to patients with mild-to-moderate COVID-19 taking this class of anthelmintics.
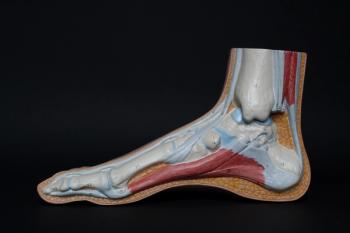
An intensive care physician offers some insights looking at fluoroquinolone-associated tendinopathy and using this social media platform to provide education and an open discussion around it.

Using a novel approach to identify E coli, investigators find approximately 8% of the bacterium’s isolates were foodborne zoonotic strains.

A new study reveals how US states fared, and some of the underlying reasons why the pandemic has played out in vastly different ways.

On this day of commemoration, The Bill & Melinda Gates Medical Research Institute has multiple TB therapies and a prophylactic vaccine in development, and its Chief Medical Officer Michael W. Dunne, MD, remains optimistic long-term about the state of clinical care, especially in areas of dire need such as low-and-middle-income countries.