Investigators report results of the first randomized clinical trial to test a novel strategy to wake up and kill dormant HIV hiding in reservoir cells.

Investigators report results of the first randomized clinical trial to test a novel strategy to wake up and kill dormant HIV hiding in reservoir cells.

PARTNER 2 study reports zero HIV transmissions over 8 years in gay couples who did not use condoms and had achieved an undetectable viral load on HIV treatment.

Scientists supported by the NIAID discover a set of broadly neutralizing antibodies in the blood of Ebola survivors capable of providing substantial protection against the disease.

National Academies of Sciences, Engineering, and Medicine workshop highlights 5 key calls for action geared at health providers responsible for treating the infectious complications of patients with opioid use disorder.

The FDA calls for safety labeling changes for fluoroquinolones to warn of the risks of mental health side effects and serious blood sugar disturbances.

Although questions remain pertaining to the E. coli outbreak linked with romaine lettuce, scientific advances led to several breakthroughs that would not have been possible just a few years ago.
A new study conducted by Tufts University School of Medicine identifies gender-specific signatures in gonorrhea infection as well as resistant genes.

The FDA has accepted an NDA and granted Priority Review for Genentech’s baloxavir marboxil for the treatment of acute, uncomplicated influenza in individuals aged 12 and older.

Stay up-to-date on the latest infectious disease news by checking out our top 5 articles of the week.
Motif Bio submits a New Drug Application for iclaprim, a targeted Gram-positive investigational antibiotic for the treatment of acute bacterial skin and skin structure infections.

Researchers from the Chang-Gung Memorial Hospital find that half of influenza cases in patients admitted to the ICU received a false-negative rapid influenza antigen test.

Researchers from the University of Mauritius identify factors that impact the growth of pathogens on kitchen towels, which can potentially result in food poisoning.
Researchers from Vanderbilt University Medical Center determine the metabolic pathway that S aureus uses to survive in bones.
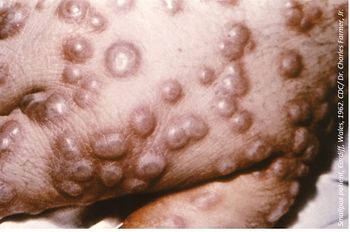
The FDA has granted Orphan Drug Designation to Chimerix’s brincidofovir for the treatment of smallpox.

As the outbreak claims more lives, the FDA performs extensive traceback efforts to identify a primary source.
Tennessee joins the list of states currently being plagued by hepatitis A outbreaks.

Stopping polio transmission will require addressing persistent challenges to vaccinating every child.
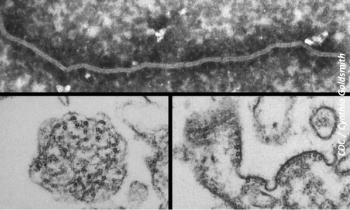
A central team led by the National Centre for Disease Control stresses that Nipah virus in India is not a major outbreak, but a local occurrence, and the situation is currently under control.

Findings from a recent study find enduring effects of LDH on learning and memory, suggesting potential clinical utility in women with HIV.
Nabriva Therapeutics plans to file a New Drug Application with the FDA in the fourth quarter of 2018.
Researchers identify a molecule found on human cells and some animal cells that could be a potential target for drugs against chikungunya virus and related diseases.

The drug met its primary endpoint of noninferiority to vancomycin at both 48 to 72 hours after drug initiation and test of cure.
Two more states have reported hepatitis A outbreaks since our last update—here’s what you should know about them.

The California Department of Public Health warns consumers to avoid the consumption of raw oysters harvested in British Columbia due to risk of contamination.

In case you missed them, we’ve compiled a list of the latest recalls posted this week.
The FDA has launched an investigation into a multistate outbreak of Burkholderia cepacia that has been linked with cleansing foam used in hospitals and home-health care settings.

An FDA inspection at Rose Acre Farms’ Hyde County Farm found rodents, insects, and poor sanitation shortly before the announcement of the multistate Salmonella outbreak.
Novavax announces that enrollment in phase 3 Prepare trial has reached approximately 4,600 participants, of whom, at least 3,000 have received the RSV F vaccine.
If clinical development progresses, the PaxVax vaccine could potentially become the first vaccine approved by the FDA for the prevention of chikungunya.
Data from the IGNITE4 phase 3 trial supports the use of eravacycline for the treatment of complicated intra-abdominal infections (cIAI), including infections caused by pathogens resistant to other antibiotics.