HCV / Hepatitis
Latest News
CME Content


Hepatitis B vaccine non-responders with chronic HCV infection were likely to respond and gain protection against HBV after HCV treatment.

Efforts to ramp up hepatitis B vaccination at birth and in infancy are crucial to controlling the disease in Africa, which accounted for 66% of new infections in 2019.

HCV infections during pregnancy increased 16-fold from 1998 to 2018 in US, with adverse maternal and infant outcomes linked to opioid epidemic.
For people living with chronic hepatitis D, the latest study results confirm the benefit of injections of this first-in-class entry inhibitor.

The CDC made this recommendation after reviewing relevant literature, and noting that complete testing jumped from about only two-thirds of patients to nearly all patients.

This day reflects the importance of remembering people who have been affected by the disease as well as a reminder to remain safe and get tested.
The phase 1/2 trial evaluating EBT-101 dosed its first patient in September 2022.
The update refreshes the organization's 2022 guidance on HIV, viral hepatitis, and STI prevention, diagnosis, treatment, and care for key populations, focusing on people in prisons and other closed settings.
A company is developing a platform to enable engineering of the first recombinant human polyclonal antibody therapies with the goal of creating a functional cure for hepatitis b (HBV).

A congressional subcommittee aims at reducing HIV-related budget items by over half a billion dollars.

In a major breakthrough, Gilead’s remdesivir (Veklury) becomes the first COVID-19 antiviral to be FDA-approved for patients with severe renal disease.

Among people living with hepatitis C virus (HCV), viral clearance was only 34%. Cure rates were even lower among uninsured persons younger than 40.

The investigational therapy from Gilead showed effectiveness and safety at 96 weeks.

Liver education has been void in the school systems and children and young adults stand to gain enormously when provided with awareness around this essential organ.

Clinicians should consider adenovirus testing in pediatric patients with hepatitis of unknown etiology. Here is an update on the incidence rates and lab diagnostics associated with identifying the virus.

Biopharmaceutical company, Vaccitech, has developed its VTP-300 vaccine, and is presenting a poster demonstrating positive early data at the ongoing European Association for the Study of the Liver (EASL) Congress 2023.

Infants age 2 to 6 months who were perinatally exposed to hepatitis C infection should be tested with a single HCV RNA test for optimal results, a recent study suggests.

While African American/Black participants were less likely to meet treatment criteria, treatment receipt did not significantly differ by race, indicating equitable access to treatment.

The agency said that in recent days a few more suppliers initiated a voluntary recall of additional select packages sold at national stores.

The first patient was dosed for this open-label trial.

With an estimated 30% of Americans living with a tattoo, the federal agency has developed guidance designed to give manufacturers information and resources to prevent potential health issues.

The new guidelines for the first time offer guidance for patients who are not fully adherent to direct-acting antivirals.

In a guest commentary, Thelma Thiel, RN, Cofounder of The Liver Health Initiative provides a personal perspective on her son’s liver issues and the importance of why everyone should get tested for HCV.
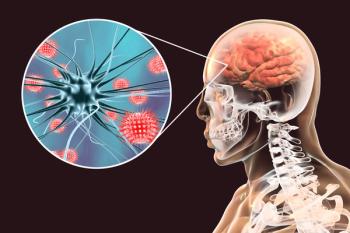
Due to worsening headaches and unrevealing cross-sectional imaging and ascites fluid analyses, this patient's differential evolved toward a central nervous system source.