Sexually Transmitted Diseases
Latest News
Latest Videos

CME Content
More News

Although preventing the transmission of HIV from mother to baby is of paramount importance, we cannot overlook other health issues that may crop up later in life for children born to women with HIV.

Brian Woodfall, MD, shares his opinion on some of the biggest advancements in HIV treatment and prevention.

What’s the 411 on 2-1-1?
A 1-month course of rifapentine plus isoniazid is non-inferior to 9 months of isoniazid alone for preventing TB in individuals with HIV, a study reports.

The new single-tablet regimen provides a safe and effective treatment option for patients with HIV.
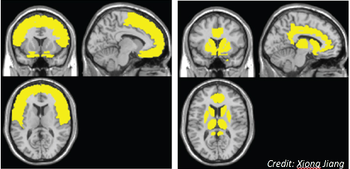
A neural model suggests the frontal lobe is affected early on in HIV disease and the caudate/striatum area is affected when neurocognitive disorder symptoms develop.
Paul Sax, MD, reacts to the ATLAS and FLAIR studies on long-acting injectable therapy.

A new study found an average decrease of sexual anxiety by 0.27 points when participants were on PrEP compared with before treatment.

Susan Swindells, MBBS, provides an overview of long-acting injectable cabotegravir + rilpivirine for clinicians.

Incorporating clinical pharmacists and stewardship program oversights into PrEP models may improve the rate of retention in care.
A team of doctors at Johns Hopkins has completed the first ever living donor HIV-positive to HIV-positive kidney transplant.

Karin Bosh, PhD, explains why the opioid overdose death rate was higher in 2015 than in 2011 among people with HIV.

With a disproportionate percentage of new HIV infections occurring in young MSM, tech tools may be a way to counter this trend.
A new report indicates that resistant isolates of gonorrhea were detected in more European countries in 2017, and may soon threaten the current recommended treatment protocol.

PrEP isn’t covered by health insurance in Germany, and non-prescription use of the HIV preventive is common. But is it safe?

A survey of MSM and transgender women found that diverse formulations and regimens for PrEP, such as long-acting injectables and “on-demand” PrEP, could increase uptake and persistence.

Julia Marcus, PhD, MPH, provides advice for health care systems looking to implement a model to identify potential PrEP candidates.

Ava Avalos, MD, details the effects of a dolutegravir-based regimen on pregnancy.

There currently aren’t any clear guidelines for how health care providers should handle discrepant—1 positive and 1 negative—HIV test results during pregnancy.
A study of MSM suggests optimizing at-home testing for STIs and HIV could increase rates of testing, while noting a need to balance convenience with educational outreach.

Paul Drain, MD, MPH, explains how point-of-care viral load testing was successful in providing rapid results to patients in a South African-based study.
A new CDC report suggests that 80% of new HIV cases were transmitted by individuals who were unaware they had HIV or who were not receiving consistent care.
The makers of leronlimab (PRO 140) have filed the non-clinical portion of the drug’s Biologics License Application as a combination therapy for HIV with the FDA.

A communitywide HIV prevention package including in-home testing and antiretroviral therapy reduces new infections, according to a study from the HIV Prevention Trials Network.