
Zoonotic & Vector-Borne Diseases
Latest News
Latest Videos

CME Content
More News

Jason Barker, ND, explains how standard testing can miss coinfections and how Vibrant’s microarray improves sensitivity and efficiency in a single test.

Sabrina Absalon, PhD, explains how antibiotic disruption of the apicoplast led to the discovery of PfAnchor, a key protein required for parasite division and survival.

A preclinical study shows the antibiotic, piperacillin, was effective at much lower dosages than doxycycline, and did so without disrupting the gut microbiome.

Sabrina Absalon, PhD, on how disrupting apicoplast division can stop malaria in its tracks and calls for sustained research funding to combat the disease.

Insights from Andrew Lover, PhD, MPH, MS, on challenges in rural Southeast Asia, border malaria, and new mosquito vectors impacting global efforts.

The Johns Hopkins Malaria Research Institute’s work in this area is looking to find ways to identify, for the first time, every single gene in the genome of malaria parasites, which could lead to the development of new treatments or vaccines.

In the first part of our interview, Sabrina Absalon, PhD, explains how depleting a protein linked to apicoplast division leads to rapid death of Plasmodium falciparum.

The Johns Hopkins Malaria Research Institute is hosting its World Malaria Day Symposium on Friday. Jane M. Carlton, PhD, offers some insights on the institute and its signature event.

Sabrina Absalon, PhD's research uncovers the essential role of PfAnchor in apicoplast division, opening avenues for targeted antimalarial therapies.

ACIP provides recommendations on meningococcal vaccines, RSV vaccination for at-risk adults, and chikungunya vaccines for travelers and laboratory workers, while also addressing safety concerns and potential expansions of use for each.

Ross M Boyce, MD, MSc, discusses the diagnostic challenges and the need for greater awareness of tick-borne illnesses in the US, considering their potential severity.

Sesh Sundararaman, MD, PhD, discusses his findings and how this approach can be triggered to minimize undesirable treatment features and open the door to newer treatment possibilities.
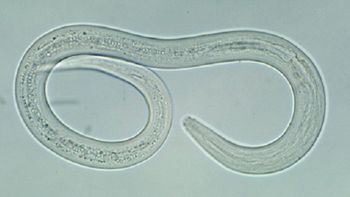
Amanda Truong, MD, PhD, provides her perspective on diagnosis, treatment, and environmental factors, considering her involvement in a recent human case.
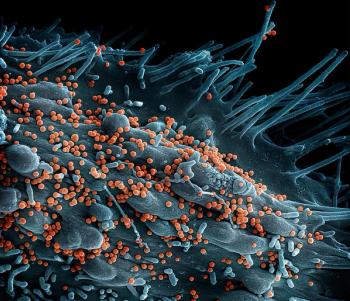
The vaccine is based on an attenuated rabies vaccine that was subsequently inactivated to make the vaccine candidate. The National Institutes of Health (NIH) is sponsoring the trial.

The phase 1/2a trial of BioNTech's BNT165e vaccine, involving nearly 180 participants, faces delays as global malaria control efforts struggle with funding and political uncertainties.

Sunil Parikh, MD, MPH, discusses the trial’s findings, noting no significant difference in malaria incidence (1.78 vs 1.84 cases per 100 person-weeks).

Sunil Parikh, MD, MPH, discussed how challenges like drug resistance and the limitations of current methods hindered ivermectin’s effectiveness in reducing malaria incidence.

Approved vaccines for chikungunya, meningococcal disease, updates on HIV prevention, key regulatory decisions, and more.

Jeff Freiberg, MD, PhD, on the rising emerging infection concerns of dengue, mpox, measles, and H5N1 influenza in clinical practice.

The approval is based on positive phase 3 clinical trial results, offering an effective option for travelers and others at risk of chikungunya, the mosquito-borne illness.

Carlien Pohl-Albertyn's, PhD research highlights the role of the South African brown locust in the transmission and survival of the emerging pathogen Candida auris.

Researchers identify Camp Hill virus, a novel henipavirus in shrews, raising concerns about human exposure to emerging infectious diseases.

Oropouche fever, primarily found in Central and South America and the Caribbean, has seen a dramatic increase in cases in Brazil.

Caryn Fenner and Petro Terblanche outline Afrigen's clinical trial plans, local partnerships, and vaccine pipeline.

Caryn Fenner and Petro Terblanche explain how CEPI’s $6.2 million grant will advance Afrigen’s mRNA vaccine for Rift Valley fever through preclinical and Phase I trials in Africa.










































































































































