An interview with a Harvard-based researcher on troubling associations between RDW and hospitalized COVID-19 patients' mortality risks.

An interview with a Harvard-based researcher on troubling associations between RDW and hospitalized COVID-19 patients' mortality risks.

Novavax made the announcement late yesterday about their investigational vaccine, NVX-CoV2373.

Adults with confirmed COVID-19 were twice as likely as control participants to report having dined out at a restaurant in the 14 days prior to symptom onset.

New international analysis highlights successes—and shortcomings—among Pacific Asia and European regions who began to reopen their societies.
Dr. Rodney E. Rohde, clinical laboratory scientist, discusses campus reopening as well as his work on rabies eradication.

The EUA grants clinicians and caregivers capability to provide serologic assay results in person to potentially infected patients.

The Chinese government approves trial for Sinovac's vaccine in these patient populations.

In the second survey in a series, more respondents said they were wearing face coverings, less would get vaccinated, and there was a slight increase in their fears about the severity of the disease.
Is it still safe to take your child to get a vaccine during the pandemic? According to experts, there are safety measures in place for this critically important task.

J&J is evaluating both 1- and 2-dose regimens of the adenovirus vector investigational vaccine.
A small study is reporting very few adverse effects and low rates of COVID-19 transmission rates from mother to baby.

A small study in Italy suggests that children are less likely than adults to be asymptomatic spreaders of COVID-19.
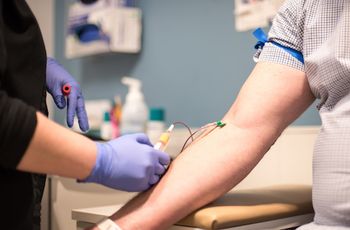
Now that men who have sex with men can donate blood after a 3-month waiting period, it’s important to determine whether the rule change is likely to increase the risk of HIV in the blood supply.

Rezafungin appears safe and efficacious in a comparison with caspofungin. The novel antifungal could facilitate easier treatment courses for patients with candidemia or invasive candidiasis.

Dr. Debra Goff describes the rush to "Just in Case" prescribing of antibiotics early in the COVID-19 pandemic, when less was known about the clinical course of the disease.

Rheumatologists are understandably concerned regarding immune suppressed patients amid the coronavirus (COVID-19) pandemic. What are the risk factors for hospitalization? And do drugs which act against inflammation complicate matters?

Medication shown to inhibit virus from infecting cells and replicating.

An estimated 450 million people suffer from hookworm infection each year, but a new study suggests it may soon be possible to vaccinate against the infection.
The time to first antibiotic dose is an important step of this new program, however it may require an audit after review according to a new study.

On Friday, new guidelines from CDC sought to show how the pandemic coronavirus (COVID-19) spreads. By Monday, those guidelines changed.

Dr. Shervin Molayem, DDS, continues our discussion on his recent paper The Mouth-COVID Connection: Il-6 Levels in Periodontal Disease — Potential Role in COVID-19-Related Respiratory Complications.

The country successfully managed thousands of mild cases among foreign construction workers at the height of the pandemic through an isolation facility. It may not be an applicable model, though.
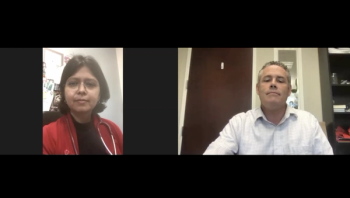
A pediatric cardiologist discusses the specifics of a case study, the importance of adaptability, and how her healthcare system has developed treatment protocols in these cases.

Medical science needs to understand the disease more clearly in this understudied patient population.
About 1 in 5 patients with bloodstream infections received discordant empirical antibiotic therapy, increasing their risk of mortality independent of sepsis or septic shock, a new study found.
What the AstraZeneca vaccine research pause meant for physician-patient discussion on vaccine candidate safety.

A quick debrief of the week’s top FDA approvals, FDA authorizations, or other infectious disease pipeline developments from the past week.

Each week, the FDA posts relevant recalls and other patient safety information. We highlight some of these notices that may be clinically relevant.

Do the norms of media coverage of SARS-CoV-2 worsen rising acute stress and depressive symptoms in the United States?

The guidance clarification published today comes just a day after reports a polarizing guidance was published last month without full review nor consensus from the agency.