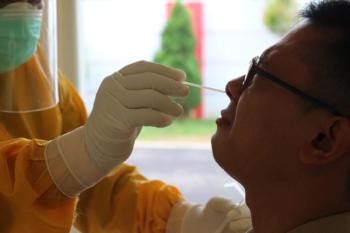
Retesting positive COVID-19 PCR tests from asymptomatic, nonexposed persons and tests stored next to a specimen with high viral load identified false positives.

Retesting positive COVID-19 PCR tests from asymptomatic, nonexposed persons and tests stored next to a specimen with high viral load identified false positives.
The National Academy of Medicine released 4 reports with COVID-19-influenced recommendations to prepare and respond to an influenza pandemic.
CEO Christopher Burns spoke about their investigational therapy pipeline, the long view perspective for the company, and how the federal government’s use of the nomenclature innovation might actually hinder incremental improvements in antibiotic development.

A new report suggests FDA and CDC deliberations may result in booster doses of the mRNA vaccine becoming available by this weekend.

Investigators analyzed data from COVID-NET across 14 states as this variant quickly become the dominant strain within the United States.
Previous research suggested vaccine efficacy significantly dips after 6 months, but a new study finds COVID-19 cellular immunity remains strong.
States with policy that permitted adolescents to independently choose to receive HPV vaccination had a significantly higher immunization uptake.

This agreement with the Medicines Patient Pool (MPP) would allow licensing of the company’s antiviral treatment candidate, PF-07321332, internationally to numerous countries.
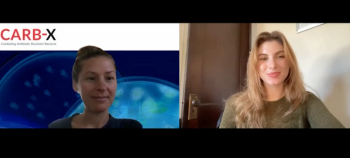
Betsy Wonderly-Trainor discusses CARB-X's mission to fund novel antibiotics, preventatives, and diagnostics in the fight against antimicrobial resistance.
Just 39 cases among more than 2.2 million doses administered.
A recent study supported the use of a therapeutic dose of heparin for moderately ill patients with COVID-19, with results showing a significant reduction in all-cause death despite no significant reduction in a composite of death, mechanical ventilation and ICU admission.
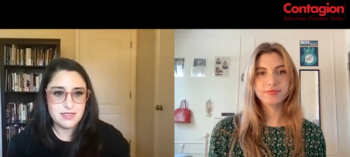
Bias against injection drug users can inhibit them from receiving potentially lifesaving care, such as HIV pre-exposure prophylaxis (PrEP). Community-based responses like syringe service programs help combat this stigma.
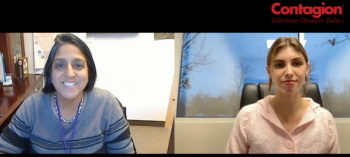
India is the world’s leading consumer of antibiotics, and Amita Gupta, MD, is researching how the country can best mitigate the spread of antimicrobial resistance.
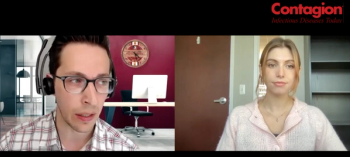
Antimicrobial stewardship efforts need infectious disease doctors, but they are in short supply. Telehealth may be the answer.
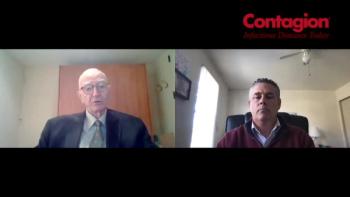
Clinical care is evolving in this area and shows promise in the various modalities and therapies that are going through clinical trials.
2 doses of the India-developed vaccine BBV152 offer 77.8% protection against symptomatic COVID-19 disease.

During the C Diff Foundation's Annual conference, providers and stakeholders offered insights on the latest investigational therapies and modalities as they advance through clinical trials.
Dr. Nicola Petrosillo discusses treating C difficile infection and maintaining antimicrobial stewardship during the height of the COVID-19 pandemic in Italy.

Adding genotypic resistance testing (GRT) to routine care did not improve HIV virologic outcomes for patients for whom first-line ART failed, the study authors wrote.

Anti-microbial resistance surveillance creates publicly accessible records that identify emerging trends in AMR and inform policy to prevent it.
Long-acting cabotegravir plus rilpivirine worked with or without a lead-in for ART-naïve HIV patients.

Social factors, such as stigma or support, strongly impacted adolescent girls and young women deciding to use HIV PrEP in Kenya and South Africa.

A multicenter, randomized trial of patients in Europe and India showed improvements in mortality among patients with COVID-19 who received a higher dose of dexamethasone.
COVID-19 testing has been an integral part of our country’s strategy to limit disease transmission. Here’s the latest information on types of tests, when they’re appropriate to use, and what they realistically can and cannot do to curb infection.
Pfizer-BioNTech's request for the Emergency Use Authorization (EUA) will apply to everyone 18 years and older.

Antimicrobial resistance is a growing problem that requires international collaboration to educate the general public and stakeholders, while simultaneously developing solutions.

A new study looks at this population and what factors affect outcomes, including diagnosis. This is significant not only for pandemic response, but also preparedness for vulnerable patient populations.

Highlights from the sessions of the World Anti-Microbial Resistance Congress 2021 included addressing the global threat for AMR and looking at real world data and real world evidence to deter resistance.

Anti-microbial resistance is one of the most critical issues in healthcare, and the CDC and WHO addressed these concerns with plans to fight back against AMR at the annual world congress.

The therapy can be used for the treatment of invasive candidiasis.