
The STOP Spillover project is aiming to prevent remerging infectious disease outbreaks.
John Parkinson is the assistant managing editor for Contagion. Prior to joining MJH Life Sciences in 2020, he has covered a variety of fields and markets including diabetes, oncology, ophthalmology, IT, travel, and local news. You can email him at jparkinson@mjhlifesciences.com.

The STOP Spillover project is aiming to prevent remerging infectious disease outbreaks.

It is important to go out into the local communities to educate people about PrEP as countries try to scale up with the World Health Organization’s (WHO) PrEP recommendations.

The World Health Organization (WHO) reports on a newly classified variant of interest (VOI) and information about another South African strain is made public. In the US, the Food and Drug Administration (FDA) prepares for meetings on COVID-19 booster doses.

The federal agency is advising people to not eat Fratelli Beretta brand prepackaged uncured antipasto trays.

The company reported its third shot induced antibody responses of more than 40 times against the Delta variant.

This virtual meeting of its Vaccines and Related Biological Products Advisory Committee (VRBPAC) will be held on September 17.

Montefiore Health System has a long history of treating infectious disease, and with the current pandemic and other emerging pathogens, they remain vigilant to potential outbreaks.
The European Council decides to remove the United States from the list of safe countries for non-essential travelers to come into the European Union (EU).

New Zealand is reporting the first death of a person shortly after the vaccine was administered.

AstraZeneca’s combination of 2 long-acting antibodies (LAAB), reduced the risk of developing symptomatic COVID-19 significantly.

The deaths occurred this month in Japan, and investigations into the deaths and the vaccine batches are ongoing.

A study from the United Kingdom shows the newer variant led to an increase in severe infections in a mostly unvaccinated population.
The Centers for Disease Control and Prevention (CDC) released data this week that showed greater breakthrough infections in a large study mainly compiled before the emergence of Delta as the predominant variant in the US.
A new report says the public health officials within the Biden Administration are preparing for making the interim period closer together.

The company announced it filed its submission to prevent COVID-19 in individuals 18 years of age and older.

August is National Immunization Month. With the FDA’s approval of the Pfizer-BioNTech COVID-19 vaccine, providers will likely be having more conversations with patients on the topic. Motivational interviewing is an emerging strategy for providers to consider in their conversations with patients who are reluctant to get the vaccine.
An extra dose generated a rapid and robust increase in antibodies.

With the full FDA approval of the Pfizer-BioNTech COVID-19 vaccine, Mark McClellan, MD, PhD, says more employer mandates will likely be coming, but he does not see federal government intervention in this area.

What does this form of pre-exposure prophylaxis (PrEP) mean in terms of options and initiation.

The decision on the final federal approval in the US could mean many more people would get the vaccine.
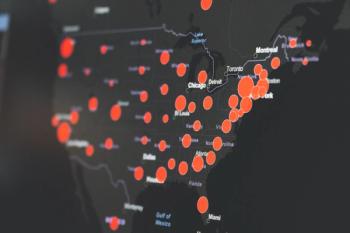
The US federal government announces plans for mRNA COVID-19 vaccine booster doses, and more children and teens are getting hospitalized for the virus.
Infection preventionists at UCLA used active surveillance screening and technology to take a proactive approach to staving off any potential outbreaks of the emerging fungal infection in their health system.

Macro and micro strategies are at play in aiming to create greater PrEP access and adoption.

The federal agency identified 3 more illnesses and the shrimp supplier expanded their recall.

The Centers for Disease Control and Prevention (CDC) endorses the additional COVID-19 vaccine shot in immunocompromised people.

The Food and Drug Administration (FDA) is expected to expand the Emergency Use Authorization (EUA) of the Pfizer-BioNTech and Moderna vaccines for a third dose in those who might be vulnerable to COVID-19.

The federal agency recommends that all women who are pregnant, recently pregnant, and planning to become pregnant get the COVID-19 vaccine.

The cases include children and adults across four states.
With the US federal government health agencies discussing plans for a booster dose for Americans and WHO’s call for a moratorium on these shots, a tale of two vaccine strategies is emerging.

Contagion Editor-in-Chief Jason Gallagher, PharmD, FCCP, FIDP, FIDSA, BCPS, discusses the quick evolution of this variant and how vaccination rates have influenced this COVID-19 strain.