
Although the numbers overall remain relatively low, the city states the rising cases as the reason for its decision.
John Parkinson is the assistant managing editor for Contagion. Prior to joining MJH Life Sciences in 2020, he has covered a variety of fields and markets including diabetes, oncology, ophthalmology, IT, travel, and local news. You can email him at jparkinson@mjhlifesciences.com.

Although the numbers overall remain relatively low, the city states the rising cases as the reason for its decision.

April 10 is a day that commemorates a population group that is emerging in terms of increased incidence rates, and needs help with inherent challenges including medication adherence and understanding of the disease.

Company is working towards full Food and Drug Administration approval in the coming months.

As this is National Public Health Week, and COVID-19 is still top of mind for clinicians and public health officials, what is it going to take for it to become a secondary issue.

This year’s theme connects climate change and health.
New data coming from Israel shows the Pfizer-BioNTech COVID-19 vaccine did offer some protection initially, but waning immunity occurs rapidly.

Being displaced by war is causing a disruption in treatment and long-term health care for Ukrainians. And it is likely, the situation will become more dire as the country’s public infrastructure worsens.

The voluntary recall issued by the company last December was investigated by federal, state and local partners.
A recent study showed that 25% of mattresses within 4 hospitals required complete replacement, underlying a serious health risk that could lead to unnecessary infections.

The Food and Drug Administration (FDA) said the company is concerned about a limited number of jars that may contain a small fragment of stainless steel from a piece of manufacturing equipment.
The Center for Global Development is launching a new working group, which aims to address antimicrobial resistance by exploring how to improve incentives for pharmaceutical companies to develop new antimicrobials for low-income and middle-income countries (LMICs).

The new action by the federal agency now means the additional booster shot can be administered to people 50 years and older.

The federal agency is expected to provide the EUAs prior to their next VRBPAC meeting on April 6.

The company is developing a single combination vaccine to cover SARS-CoV-2, influenza, and respiratory syncytial virus (RSV) as well as another candidate for endemic human coronaviruses.

This designation of the company’s respiratory syncytial virus (RSV) vaccine candidate, PF-06928316, will help to expedite its development and review.

Along with reporting positive data in the youngest pediatric population, the company is working with the Food and Drug Administration (FDA) for emergency use authorization of its vaccine in children 6 years to under 11 years as well as the 12-17 age groups.
A new virtual program aims to train clinicians and other providers on terminology and how to engage patients in effective dialogue and counseling.

The submission is for a fourth dose of its Spikevax (mRNA-1273) vaccine in adults 18 years of age and older.

The application is based on 2 Israeli studies for the additional shot for people 65 years and older.

This study comes just weeks after the company announced another HIV vaccine trial.

In a nationally televised interview, Pfizer Albert Bourla said it is necessary to fend off waning protection.

The phase 3 results for its antibiotic, cefepime-taniborbactam, demonstrated it met its primary endpoint and the company says it is on track for a fourth quarter 2022 FDA NDA.

The company is examining its antiviral pill for children 6 to 17 years of age in combating COVID-19.
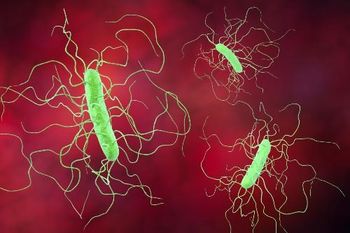
The company’s investigational PF-06425090 vaccine did show benefits including decreased median infection period and no hospitalizations in the vaccinated cohort.
This week, one of the m-RNA COVID-19 vaccine’s effectiveness was shown to drop from 68% to 12% in children 5-11 years old within a month. This data raises concerns of what should be done to protect this pediatric population.

The federal agency cited chemistry manufacturing and controls issues with vials.
New research points to vaccine effectiveness against infection dropping from 68% to 12% in this pediatric population within a month.
Although it is possible, a small Danish study showed the majority of these cases were often mild and in unvaccinated individuals.

This is broken down into counties that are either high, medium, or low level categories. Many areas of the US are able to go without masks.

The federal agency is going to lay out recommendations for states.