The award given to the research institute by the state of Pennsylvania looks to delve deep in understanding vulnerable populations and taking some lessons away in addressing future potential pandemics.
John Parkinson is the assistant managing editor for Contagion. Prior to joining MJH Life Sciences in 2020, he has covered a variety of fields and markets including diabetes, oncology, ophthalmology, IT, travel, and local news. You can email him at jparkinson@mjhlifesciences.com.

The award given to the research institute by the state of Pennsylvania looks to delve deep in understanding vulnerable populations and taking some lessons away in addressing future potential pandemics.

The Advisory Committee on Immunization Practices (ACIP) voted yes to a third shot of the Pfizer-BioNTech COVID-19 vaccine in this pediatric population.

A pregnant woman is reported to be the country’s first person to be infected simultaneously with COVID-19 and influenza.
The country says one city hospital’s data shows a decrease in severity and mortality during this latest variant's outbreak.
Investigators in the United Kingdom showed a Pfizer-BioNTech booster dose increases neutralizing antibodies in a laboratory setting, and Omicron might not be as damaging to the lungs as previous variants.
The fast-moving Omicron variant demonstrates a high transmissibility, but not as significant a hospitalization rate or mortality.
A phase 3 trial shows one dose is 91.7 % effective against severe disease.

Stigma, and a lack of communication and awareness all inhibit HIV PrEP uptake, but digital health and new forms of PrEP are aiming to increase users.

This is the first pill to treat COVID-19 in trying to prevent progression to severe disease and possible hospitalization.

The administration is going to buy 500 million at-home rapid COVID-19 tests, and make them available to Americans. They also plan to use federal resources to provide pop-up clinics and get more pharmacists involved in vaccinations.

The therapy becomes the first injectable approved in the United States for this indication.
The proposed funding will include a number of measures of spending towards HIV and prevention, including community centers for PrEP education.

The website, LearnAntibiotics.com, is a supplemental tool offering guides, practice exams, and other resources to help students, residents, or practicing professionals with infectious diseases pharmacotherapy.

The company believes the pill should work against the Omicron variant.

Data indicate that a third dose of the Pfizer-BioNTech vaccine, BNT162b2, increases the neutralizing antibody titers by 25-fold compared to 2 doses against the Omicron variant.

The strategies address booster doses, travel, and rapid response.
The first Omicron COVID-19 case was discovered in California. The patient, who traveled from South Africa, has since recovered.

As there is a lot of information regarding the Omicron variant, here is our latest slideshow to offer some insights on it.
Moderna and Pfizer's CEOs voice doubt on the current vaccines’ efficacy with the new variant, but are developing prophylaxis vaccines and treatment strategies to curtail the new strain.

The strain identified as B.1.1.529 is causing some travel restrictions from some African countries, and medical science is rushing to understand its potential impact on the global stage.

HIV Stakeholders weigh on the future of PrEP.

The federal agency's decision makes this the first CMV treatment for this patient population.
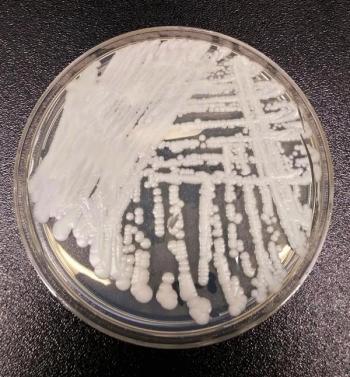
This infection has the ability to remain undetected and the potential to spread very quickly in long-term care facilities and hospitals.

As providers were challenged to care for patients with severe COVID-19 In the first year of the pandemic, antibiotic usage increased. Listen to clinicians talk about this specific challenge and how prescribing practices evolved over time.
From its origins as a serendipitous discovery to being successfully prescribed for nearly a century, antibiotics remain a foundation of medicine. However it has major ongoing challenges including the rise of superbugs and the development of antimicrobial resistance (AMR). There are some prospective strategies to combat AMR on the horizon.

Providers and key stakeholders weigh in on this significant topic.
CEO Christopher Burns spoke about their investigational therapy pipeline, the long view perspective for the company, and how the federal government’s use of the nomenclature innovation might actually hinder incremental improvements in antibiotic development.

This agreement with the Medicines Patient Pool (MPP) would allow licensing of the company’s antiviral treatment candidate, PF-07321332, internationally to numerous countries.
How 1 provider is training medical peers to offer PrEP education and prescribe it.

Fenway Health’s Pride in Our Health podcast is an example of new voices being heard, and an alternative means of getting health information to the general public.