
The federal agency is going to lay out recommendations for states.
John Parkinson is the assistant managing editor for Contagion. Prior to joining MJH Life Sciences in 2020, he has covered a variety of fields and markets including diabetes, oncology, ophthalmology, IT, travel, and local news. You can email him at jparkinson@mjhlifesciences.com.

The federal agency is going to lay out recommendations for states.

This is the first authorized COVID-19 vaccine that was developed by a Canadian-based company, and the first that used a plant-based protein technology.

Pfizer’s TicoVac vaccine was developed to immunize young children and adults to prevent Tick-Borne Encephalitis.
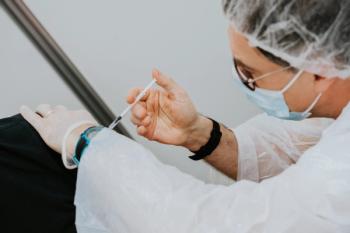
The companies plan to seek regulatory authorization for the vaccine both in the US and Europe.
A clinician offers his perspective on how the virus is behaving and the role that vaccines and therapies will play.
A preprint study shows the variant is resistant to some monoclonal antibodies and vaccines.
Investigators looked at myocardial infarction risks over several years within 2 health care systems examining both people with HIV (PWH) and people without HIV (PWoH).
The investigational therapy has demonstrated promising results utilizing subcutaneous injections every 6 months.
For people living with HIV (PLWH) an accelerated aging process may affect the severity of their disease.
Investigators looked at full vaccination and booster dose observational data to offer some insights on protection and breakthrough infections.

This patient is the first mixed race woman to experience HIV-1 remission, and potentially opens up the door to others being treated with a novel treatment.

Investigators found a couple of different factors for this phenomenon.

Data shown in the Pinetree clinical trial and being presented with 2 posters at CROI demonstrate both benefits and safety in preventing progression to more severe disease.

Investigators looked to see if this population might be candidates for this form of HIV prevention.

Boston’s Fenway Health looked at testing and prevention trends during 3 phases of the COVID-19 pandemic.

This decision was based from the analysis of the company’s phase 2 BLAZE-4 trial.

In this latest episode of Contagion Community, we interview Dr. Jacinda Abdul-Mutakabbir about vaccine equality vs vaccine equity as well as her experience on a COVID-19 vaccine drive to increase vaccinations in communities of color.

Their investigational vaccine, NVX-CoV2373, demonstrated 82% efficacy against the Delta variant in participants 12-17 years old.

A study looked at COVID-19 vaccination rates and new COVID-19 infections across US counties during the Delta surge last summer. Many of the highly infected counties were considered rural, highlighting some of the vaccination challenges surrounding these areas.
A process known as thin film freezing can convert existing therapies into powder that offers another delivery solution and may help alleviate storage challenges in developing countries.
The company recently submitted its data to the federal regulatory authorities for an Emergency Use Authorization of its COVID-19 vaccine, NVX-CoV2373. As it's time to think about this potential vaccine being available in the US, clinicians offer some insights and perspective on it.

The Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) voted 13-0 in favor of the vaccine for adults.

A sub-analysis of phase 2 data compared the immunogenicity of their VLA15 vaccine in adults after administration of 2 or 3 primary series doses with the latter demonstrating a stronger response.

The company’s Spikvax vaccine was approved based on its submission that included efficacy and safety data approximately 6 months after second dose.

Company announced it had administered its first shot to a participant for its extension of an earlier phase 2 study.
The companies said the vaccines will be examined in adults 18-55 years old.

More than 1.2 million people were estimated to have died in 2019 as a direct result, and that antimicrobial-resistant infections played a role in millions more.

In utilizing a modeling study, investigators estimated reductions in HIV incidence rates as well as PrEP usage rates and viral suppression across US cities over 10 years.
A predictive tool, POC Advisor, has been developed to support faster diagnosis and intervention of this dangerous condition.

During a continuation of a difficult time in our history, overwhelmed providers need to take a step back and find time for themselves to unwind and destress from COVID-19.