
Nina Cosdon
Nina Cosdon is the associate editor for Contagion. Before joining MJH Life Sciences, she graduated magna cum laude from Denison University in 2021 with a degree in Communication. You can find her reading, hiking, or antiquing, or by emailing her at ncosdon@mjhlifesciences.com.
Articles by Nina Cosdon


In hospitalized COVID-19 patients, remdesivir stewardship reduced hospital length of stay and therapy duration.

The CDC’s COVID-19 booster dose recommendation for children 5 and older comes a few days after the FDA’s authorization

Among community-acquired pneumonia (CAP) outpatients, 49% were prescribed unnecessary antibiotics.
Leveraging antimicrobial stewardship programs was crucial to ensure COVID-19 hospital inpatients received monoclonal antibody therapy quickly and safely.

To reduce the risk of antimicrobial resistance, rapid laboratory diagnostics are needed to identify the pathogens in hospital patients with COVID-19 and sepsis.

The FDA lifted a clinical hold on investigational lenacapavir for HIV treatment and pre-exposure prophylaxis. All clinical studies evaluating injectable lenacapavir can now resume.

Today, the International AIDS Vaccine Initiative (IAVI) and Moderna announced they will soon launch a phase 1 clinical trial for their HIV vaccine candidate, administered with mRNA technology to develop broadly neutralizing antibodies.

A third, “booster,” dose of the Pfizer-BioNTech COVID-19 vaccine is now FDA-approved for children 5-11 years old.

Though it failed to meet its primary study endpoints, post hoc analysis of SNG001 showed the inhalable reduced the risk of severe COVID-19 disease and death in high-risk patients.
Stage 2/3 trials are underway to test the efficacy of J08, a potent monoclonal antibody, to prevent and treat current and future COVID-19 variants.

COVID-19 patients who do not speak English faced worse hospital outcomes during the first wave of the pandemic.

A study of pregnant women positive for COVID-19 at delivery found no evidence of vertical transmission, but increased gastrointestinal problems in their newborn infants.

A comparison of COVID-19 PCR tests and rapid self-tests found self-tests to be highly accurate and user-friendly for children.
In Part 2 of a video interview, Sornana Segal-Maurer, MD, discusses the newly published research findings that detail the potential of lenacapavir.

Could lenacapavir be the answer for people with multidrug-resistant HIV infection? Principal investigator Sorana Segal-Maurer discusses the promising trial results in this challenging cohort (Interview part 1).

At 1 year after COVID-19 infection, children’s neutralizing antibodies only differed by vaccination status.

Despite a significantly slower than expected COVID-19 vaccine rollout, Novavax reports early 2022 as its first profitable quarter.
The higher the gestational age when pregnant women received a COVID-19 vaccine, the higher the serological titers at birth.

Children living with HIV had a similar immune response to mRNA COVID-19 vaccination as children without HIV.
Investigators examined whether rapid antigen tests were less sensitive to variants of concern. Though the tests detected Omicron, all but 1 were less effective in detecting the Delta variant.

A member of the herpes family, Epstein-Barr virus is the primary cause of infectious mononucleosis and may also cause certain cancers and autoimmune diseases.
Investigators identify 11 crucial “protection-defining genes” that prevent a severe disease response to COVID-19 infection.

Medicago’s plant-based vaccine, Covifenz, was 69.5% effective against symptomatic COVID-19 infection and 78.8% effective against moderate-to-severe disease.

HIV reservoirs can remain dormant, reigniting infection at an opportune moment. New strategies seek to reignite these viral reservoirs so they can be identified and destroyed by the immune system.

Antibiotic-resistant strains of the dangerous superbug C difficile have been identified in pigs and humans, suggesting zoonotic transmission is possible.

Today, the FDA approved vonoprazan dual and triple therapies, developed by Phathom Pharmaceuticals to treat adults with H. pylori infection.
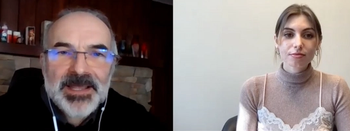
Dr. Simon Portsmouth of Shionogi discusses the trial results of their once-daily oral COVID-19 antiviral, S-217622, as well as the significance of the therapy as vaccine efficacy wanes in the age of Omicron.

Utilization of ambulatory care services declined during the COVID-19 pandemic, especially among socioeconomically disadvantaged Americans.
