
A systematic review of clinical trials found a high probability that fluvoxamine prevented COVID-19 hospitalizations in outpatient settings.
Nina Cosdon is the associate editor for Contagion. Before joining MJH Life Sciences, she graduated magna cum laude from Denison University in 2021 with a degree in Communication. You can find her reading, hiking, or antiquing, or by emailing her at ncosdon@mjhlifesciences.com.

A systematic review of clinical trials found a high probability that fluvoxamine prevented COVID-19 hospitalizations in outpatient settings.
The risks of deep vein thrombosis, pulmonary embolism, and bleeding events were increased at 3 or more months after COVID-19 infection.

In Germany, HIV pre-exposure prophylaxis users are recommended to receive regular HIV, STI, and renal function testing. How frequently are PrEP users getting tested?

The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) met today to discuss how to proceed with informing future COVID-19 vaccine strain composition and booster decisions.

After the US Food and Drug Administration (FDA) fully authorized the Pfizer-BioNTech mRNA vaccine, series-completing second doses increased substantially. However, first vaccine doses were actually administered at lower rates after the approval.

A new study found people who staunchly oppose COVID-19 vaccines were more likely to have experienced adverse childhoods, making them distrustful from a young age.
Notoriously slow to implement new practices, most hospitals rapidly updated their standard treatment procedures during the COVID-19 pandemic.

Using CRISPR technology, investigators were able to “knock out” primary human CD4+ T cells, identifying new genes essential to HIV viral replication.

Children under 5 became infected with the Omicron variant 6-8 times more frequently than young children who contracted Delta. However, Delta COVID-19 infections were more severe than Omicron.

Despite lower comorbidity risk scores than Black or White COVID-19 patients, Mississippi’s Indigenous populations had significantly higher in-hospital mortality rates.

One day after the FDA approved second booster shots for certain vulnerable populations, the National Institute of Health (NIH) announced they have begun enrolling adult US participants in a phase 2 clinical trial to evaluate a second COVID-19 booster dose.
2 doses of the Pfizer-BioNTech vaccine reduced Omicron hospitalizations by 68% in children 5-11 and by 40% in adolescents 12-18 years old.

Today, the FDA approved the abacavir, dolutegravir, and lamivudine single tablet formulation Triumeq, a once-daily HIV treatment for children.

The Pfizer-BioNTech and Moderna mRNA vaccines were found to produce different antibody and killer T-cell responses, suggesting a “mix and match” booster approach may provide the best protection against COVID-19.
“Together these 2 diseases create a perfect storm”: Black people with cancer were more likely to have severe or fatal COVID-19 disease.

Today, the FDA approved the long-acting HIV treatment Cabenuva (cabotegravir and rilpivirine dually administered) for virologically suppressed adolescents 12 years and older.
The coronavirus pandemic caused the first global recorded decline in human life expectancy.

The Assistant Secretary for Preparedness and Response (ASPR) announced a pause in sotrovimab distribution in regions where BA.2 is the dominant COVID-19 variant, citing evidence that the monoclonal antibody therapy would not effectively neutralize the BA.2 Omicron variant.
Tori Pipak, a physician assistant at Allegheny Health Network’s Positive Health Clinic, details the comprehensive services the clinic provides to people living with HIV.

Treating long-lasting nets with a new insecticidal combination that rendered mosquitos unable to fly reduced malaria infections in Tanzanian children by almost half.

People with immune dysfunction are at increased risk for COVID-19 breakthrough infection after vaccination, and should take additional precautions.
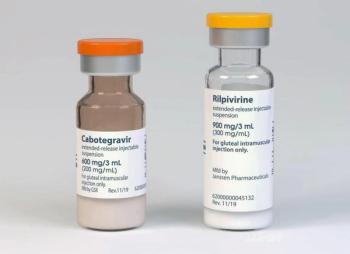
With FDA Approval, the oral lead-in for the cabotegravir and rilpivirine long-acting HIV treatment (Cabenuva) is now optional. Clinical trial data showed similar safety and efficacy profiles for initiating the therapy with or without the oral lead-in.

Congress’s spending bill allocated drastically less funding for HIV testing, prevention, and treatment than previously promised. Carl Schmid, executive director of the HIV+Hepatitis Policy Institute, explains why it is so vital to continue the fight against HIV.

Research set to be presented next month found that people with resolved COVID-19, and in some cases active COVID-19, could safely donate organs.

Here’s a roundup of popular foods and products that were recalled this week by the US Food and Drug Administration (FDA).

Series-specific antibiotics can be far less effective if there are other microbes present.

A matched cohort study found COVID-19 infection increased the risk of new type 2 diabetes diagnosis. Compared to patients with acute upper respiratory tract infections, COVID-19 patients were 28% more likely to develop diabetes.

There are many unknowns about the BA.2 COVID-19 variant. It appears 50-60% more transmissible than the original Omicron variant, but health experts are mixed on whether cases will spike dramatically.

A Texas study found children and adolescents who previously contracted COVID-19 retained protective antibodies for 6 or more months after infection. However, natural infection plus vaccination remains the best defense against COVID-19.

People who use drugs face obstacles, like homelessness and stigma, that hinder their access to healthcare. Dr. Brianna Norton opened the Montefiore-NYHRE Clinic, a first-of its kind academic medical center, to serve marginalized people holistically and respectfully.