The novel variant was found to be 1.7 to 2.4 times more transmissible and can evade 10% to 46% of gained immunity.

The novel variant was found to be 1.7 to 2.4 times more transmissible and can evade 10% to 46% of gained immunity.
With a 75% vaccination rate, cases, hospitalizations and ICU administrations will plummet.

The company will provide the vaccine group with 34 million doses in the fourth quarter of this year, and up to 466 million more doses in 2022.

A report said the vaccine may receive a US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for the 12-15 year old population as early as next week.

The Connecticut, New York, and New Jersey governors announced plans to return to full capacity again.
Antibody levels induced by mRNA COVID-19 vaccines are much higher than those induced by natural infection and confer cross-reactivity that could be effective against new variants, a new study from the University of California, Irvine, found.
Aid shipments begin to arrive in India from the United States and Britain in efforts to help the country.

A treatment arm of up to 3000 participants aged 12-17 years old will be rolled into the North American-based PREVENT-19 trial.
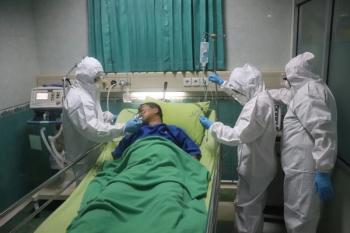
Understanding the mechanisms driving inflammation related to IL-6 should be prioritized over rushing to treatment, especially in the era of COVID-19, the study authors said.

The multidisciplinary, retrospective analysis showed that long-term outcomes of ECMO patients did not vary from that of others.

The US will initiate travel restrictions beginning May 4th, due to the surging COVID-19 case load in India.
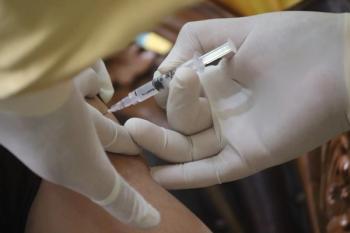
The report details what symptoms vaccinated people should look out for, as well as treatment details for healthcare professionals.

While a slightly higher rate of morbidity was observed, a small percentage of babies presented with COVID-19 after birth.
A prolonged infection can allow a virus to mutate more often, potentially letting more transmissible variants to emerge.

Pre-existing conditions were found to increase the risk of COVID-19 related death in those with diabetes.
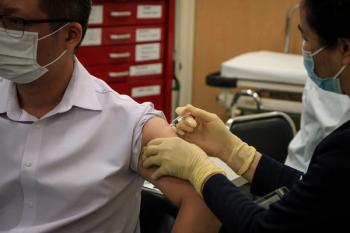
The confusion and uncertainty surrounding the need for a second dose among the public shows the need for better guidance from the medical community.

Patients who received the inhaled glucocorticoid budesonide in the first several days of COVID-19 symptoms were much less likely to require hospital care.
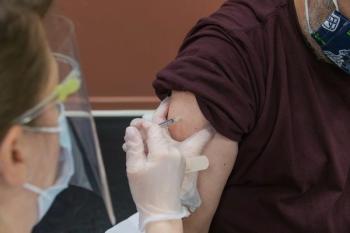
The most common symptoms were headache, fatigue and soreness at the injection site.

A pair of recent studies suggest that the ability of people with blood cancers lymphocytic leukemia and multiple myeloma to produce antibodies in response to COVID-19 vaccines is impaired.

Surveys suggest most students plan to get the shots anyway.

The discovery of these nanobodies may help to create treatments that can protect against COVID-19, as well as future variants of the virus.

Individuals who are fully vaccinated can shed their masks when exercising outdoors and gathering in small groups.

Exposure to a segment of the viral spike protein induced COVID-19 like symptoms and caused lung damage similar to those with the disease.

Even in mild cases, symptoms of COVID-19 can linger for at least 8 months, according to new research.

The Biden Administration plans to donate the AstraZeneca vaccines globally upon clearance of safety reviews.