
These were our most popular articles this week. Catch up with these trending infectious disease stories.

These were our most popular articles this week. Catch up with these trending infectious disease stories.

“Too few persons are receiving timely treatment” with direct-acting antiviral for hepatitis C based on their first positive test result, the study authors wrote.

The FDA cleared zetomipzomib as an Investigational New Drug. A phase 2 clinical trial is the next step for this autoimmune hepatitis treatment.
The intervention included meeting with experts, texting services to improve and facilitate communication, and data collection and dissemination.

The federal agency said it was looking at a multistate outbreak of the infection connected to Brie and Camembert soft cheese products manufactured by Old Europe Cheese.
Oral antibiotics after partial completion of intravenous regimen for S aureus bacteremia improves outcomes of persons who inject drugs (PWID), which is a population that often has limited access to treatment.

The CDC is urging all eligible persons to receive a flu shot, but a new survey shows fewer adults are planning to get the vaccine this season.
Bulevirtide, a first-in-class entry inhibitor, showed promise for the treatment of hepatitis B and hepatitis D coinfection, a phase 2 trial found.
Yasaswi Kislovskiy, MD, MSc, specialized in reproductive infectious disease to advocate for gender equity in health care.

Experts are advocating for occult hepatitis B to be spotlighted.

With proper treatment, even the youngest sufferers can recover. Know the signs when treating potentially infected children, especially if they have environmental risk factors.

Largest extended study of outcomes after rare myocarditis from mRNA COVID-19 vaccines finds most recover and regain quality of life.

Catch up on Contagion's top stories from the month of September.
“Vaccines are the most highly scrutinized public health interventions we know,” said Donald Alcendor, PhD.
The federal agency recommends using either PCV13 or PCV15 as part of a 4-dose series for pediatric patients between the ages of 2-59 months.
The investigational therapy demonstrated time to first resolution of the five COVID-19 symptoms was significantly reduced in those treated with low dose of medication.

C diff patients live in fear of a recurrence, says gastroenterologist Paul Feuerstadt, MD. RBX2660 has entered the FDA pipeline and looks to end this vicious cycle.

A European panel of experts endorsed the FDA’s recommendations.
Interim data show a bivalent vaccine that contains the Omicron variant may be more effective.
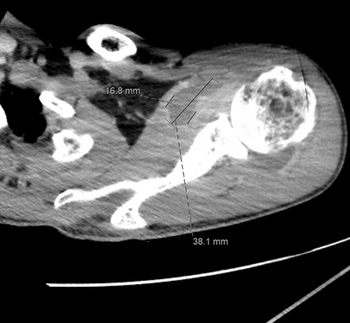
Gonococcal septic polyarthritis is an uncommon manifestation of N gonorrhoeae infection, but has increased by about 40% in recent years.
Uganda’s first Ebola outbreak in a decade raises concern due to its vaccine resistance.

Occult HBV infection is likely to go undetected in under-resourced regions of high HBV endemicity and confound WHO goal to eradicate the viral hepatitis by 2030.
With an all-ready challenging patient population to treat, having a program to get more people into treatment can be helpful in an underserved group.

The limitation in the number of pediatric-specific guidelines by professional organizations, and results from large randomized clinical trials, may place an additional emphasis on literature evaluation skills for pediatric ASP pharmacists.
A fecal microbiota transplant (FMT) trial was halted early due to clear evidence the treatment was superior to placebo.
These vaccines target the wild strain as well as the Omicron BA.4/BA.5 variants.

Among pregnant women coinfected with HIV, a higher hepatitis C viral load increased the likelihood of transmitting HCV to children.

Recent issues surrounding the ADAPT-PO and SURE-2 trials place the spotlight on the difficulties developing oral carbapenem-based antibiotics and case their future into doubt.
Investigators examined all-cause excess mortality and sought to uncouple it from COVID-19 in US states with high vaccination rates.

Similar to HIV, the colonial and postcolonial history is fundamental to the biology of this monkeypox outbreak.