
The federal agency's recommendation for both the Moderna and the Pfizer-BioNTech updated bivalent vaccines allows people to get the booster dose this fall.
John Parkinson is the assistant managing editor for Contagion. Prior to joining MJH Life Sciences in 2020, he has covered a variety of fields and markets including diabetes, oncology, ophthalmology, IT, travel, and local news. You can email him at jparkinson@mjhlifesciences.com.

The federal agency's recommendation for both the Moderna and the Pfizer-BioNTech updated bivalent vaccines allows people to get the booster dose this fall.
Taking into account the first PrEP pill was FDA approved in 2012, a clinician reflects on its significance and where the shortcomings of education and utility of it needs to be fortified.

This Emergency Use Authorization for the Moderna and Pfizer-BioNTech vaccines brings them both one step closer for people to become eligible for these boosters.
A biotech company is utilizing this platform to rid people of the virus using a technology that differs from CRISPR-Cas9.

The Cambridge-MA., company accuses Pfizer and BioNTech of copying patented technology in the development of their COVID-19 vaccine.

From its results, the company plans to file a Biologics License Application (BLA) with the FDA.

If granted the Emergency Use Authorization (EUA), this vaccine would be available for individuals 18 years and older.

The companies are requesting the Emergency Use Authorization (EUA) for 30-µg booster dose of an Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine for individuals 12 years of age and older.

If it is granted an Emergency Use Authorization (EUA), the vaccine could be used as a booster dose for individuals 18 years and older vaccinated with any of the other available COVID-19 vaccines.

The booster is designed for immunization against the original wild strain as well as Omicron variant.

The agency decided to allow for intradermal injection thus increasing the number of available doses substantially.

The company and its partner, Pfizer, are preparing for the potential launch of 2 variant-adapted bivalent COVID-19 vaccines assuming authorizations from FDA and EMA.

The latest MMWR offered guidance to these high-risk groups.

A single case was identified last month, and wastewater samples shows the potential for more cases.
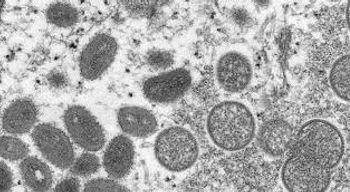
The federal agency and international organization offer some insights on patient epidemiology, and the high-risk groups that public health officials and clinicians should consider for vaccines and education efforts.

The findings come from a large Dutch study and demonstrate the symptoms of the virus months after people's acute infections.

Declaration allows mobilization of funding, resources to address the virus.
A new study finds there are more pharmacies than physician practices in these areas; however, these inequities offer an opportunity for pharmacists to step-in and provide vaccine education and administration.

This study looks to identify safety, efficacy of the vaccine in the youngest pediatric populations.
The benefits outweigh the possible rebound of symptoms, according to an investigator who studied the antiviral.

The modified spike protein in this next-generation bivalent candidate is part of the companies' strategy to try and provide vaccines with longer and more durable protection against the virus.

The aim is to increase vaccination rates and awareness in the state with the second most confirmed cases.

This regulatory review is for the prevention of recurrent vaginal yeast infections. And if approved, it would be a second indication for the antifungal related to these infections.

Public health officials made the decision after seeing increasing incidence rates.
Working to Fight AMR is working in Washington DC to help pass policies in Congress to help alleviate this significant issue.

He is experiencing mild symptoms and is going to take Paxlovid.

This decision follows votes to allow the vaccine to be indicated for prevention of the virus in adults 18 years and older.

A specific immunization marker showed the percentage of children receiving common vaccines fell 5% between 2019 and 2021.

Federal government agencies offer a best practices approach for testing, announce more laboratories are testing for the virus and provide insights on vaccines and therapies.

The European agency says there have been “a few cases of anaphylaxis.” The label will also include warnings for paraesthesia and hypoaesthesia.