Contagion® caught up with William Schaffner, MD, medical director of the National Foundation for Infectious Diseases, to discuss the highlights from this week’s NFID Annual Conference on Vaccinology Research.

Contagion® caught up with William Schaffner, MD, medical director of the National Foundation for Infectious Diseases, to discuss the highlights from this week’s NFID Annual Conference on Vaccinology Research.

An intradermal Ebola vaccine candidate shows promise as a safe, temperature-stable, and effective option for preventing outbreaks, new data show.

The latest case cluster in the United States has led a university to change policy regarding the MMR vaccine.
To shed light on the current measles outbreaks, Contagion® is launching a video series featuring the perspectives of 2 medical experts.




Countries around the world begin to respond to measles outbreaks, with Italy taking the step of keeping unvaccinated kids home from school.
With 206 cases of measles reported so far in 11 states in 2019, a new study noting that there is no connection between the measles, mumps, rubella (MMR) vaccine and autism has been released.
The rise of Shingrix as a first-choice prophylaxis for shingles has lead to increased demand as the manufacturer commits to ramping up production.

Vaccine-preventable disease outbreaks are on the rise and social media has a responsibility to help rein it in
With 6 ongoing outbreaks, cases reported across 10 states, and the FDA commissioner contemplating federal intervention, measles remains at the forefront of collective consciousness.
As flu activity remains elevated in all regions of the United States for the fifth week in a row, a new study quantifies the impact of flu vaccination last season.
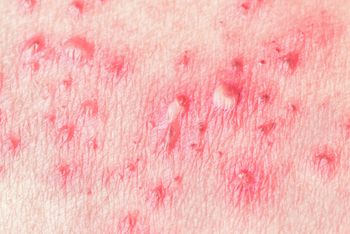
Post-licensure safety data on recombinant zoster vaccine (Shingrix) is consistent with pre-licensure clinical trial data, although the CDC cautions that the immunization is still in the “early uptake period.”
The FDA approved the vaccine in 2016 for adults between the ages of 18 and 64 who are traveling to areas of active cholera transmission.
With early season flu vaccination rates up from 2017-2018 early season rates, new pediatric influenza deaths around the country are prompting health officials to call for even higher vaccination rates.
The vaccine is approved as a 3-dose series, which consists of a 0.5 mL intramuscular injection, administered at 2, 4, and 6 months of age.
TY014 is the first candidate drug to be evaluated in clinical trials for the treatment of yellow fever.
The results of a new Dutch study suggest a rationing protocol does not diminish the effectiveness of the vaccine.

A new survey has found that 34% of US parents are not planning to have their child vaccinated against influenza.

Investigators have found that children who received a flu shot in consecutive years did not see a decline in vaccine effectiveness.
Keeping communities safe from potentially deadly viruses is everyone’s responsibility.

New outbreaks of rubella and troubling data on influenza highlight the importance of vaccination.