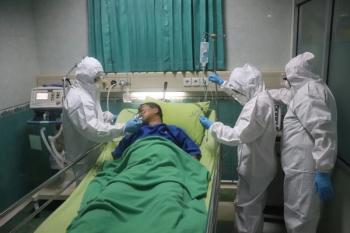
A 2-year retrospective study looking at patients admitted to the ICU showed that the number of intubated males infected with Acinetobacter baumannii (AB) was double the number of intubated females.

A 2-year retrospective study looking at patients admitted to the ICU showed that the number of intubated males infected with Acinetobacter baumannii (AB) was double the number of intubated females.

Recent numbers show a lack of new people entering the field combined with existing shortfalls in many US locations.These factors demonstrate a potentially dangerous reduction in this vital medical specialty.
Clostridioides difficile (CDI) and recurrence can create a tremendous burden on patients’ quality of life as well as become a financial burden to individual healthcare systems thus creating downstream costs for individual hospitals.
After their first Sudan ebolavirus outbreak in 10 years, resulting in 164 cases, Uganda today announced the Ebola outbreak is over.

The agency licensed the new indication for Sanofi’s Adacel (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Adsorbed [Tdap]) shot for immunization during the third trimester of pregnancy.

Hong Kong confirmed a case of a man critically ill with avian influenza. On the other side of the world, Colorado is reporting its worst-ever avian flu outbreak among birds of prey.

Research will focus on new technologies for early diagnosis of severe illnesses resulting from SARS-CoV-2 infection.
RSV cases spiked to unprecedented levels this season. Why were these infections so frequent and severe? Why has vaccine development taken so long?

They developed clinical guidance and other resources looking to address potential drug interactions with these patient populations who have COVID-19 and may be treated with Paxlovid.
Good news: across 23 countries, trust in COVID-19 vaccines increased 5.2% from 2021 to 2022. However, vaccine hesitancy remains in key populations.

The company is moving forward with vaccines to address respiratory and latent viruses as well as oncology.

Evusheld, the only FDA-approved COVID-19 pre-exposure prophylactic, is anticipated to be ineffective against the emerging XBB.1.5 variant.

The investigational antiviral showed the potential of another therapy that could benefit this patient population that is at risk for hospitalization and death.

Newer diagnostic technologies can play a role in identifying specific bacterium, decrease the time to optimal therapy, and offer the possibility of better outcomes.

A Brazilian study of people 90 years and older who easily recovered from COVID-19 identified specific genetic components that may have provided protection.

The VIASURE PCR diagnostic is now available under Emergency Use Authorization in laboratories for persons with a suspected mpox infection.
Central Ohio has seen 82 pediatric cases since November, and more than a third of children infected have been hospitalized.

So far, these are Contagion's 5 most-read stories of 2023.

Developed by Sanofi and AstraZeneca, the single-dose long-acting antibody was designed to help protect all infants from birth through their first respiratory syncytial virus (RSV) season.

The 24-valent investigational pneumococcal conjugate vaccine, VAX-24, met its primary endpoints in a phase 1/2 trial.

A new Infectious Diseases Society of America (IDSA) board member and vice president offers some insights on the leadership’s goals and direction for 2023.

The company’s vaccine candidate, MenABCWY, which is indicated for adolescents was given a Prescription Drug User Fee Act (PDUFA) goal date for later this year.

In just a month, the XBB.1.5 variant went from causing 1% to over 40% of new COVID-19 infections.

Due to negative results, the I-SPY COVID Trial is no longer evaluating cyproheptadine to treat critically ill COVID-19 patients.

In the latest SIDP column, two clinicians provide information on recent studies and guidelines directed at antibiotic durations in this patient population.
Health programs and efforts to inform the public about the importance of sharing liver education can play a major role in prevention as well as patient care.

During the COVID-19 pandemic, cigarette sales differed from projected sales volume. One study examined these trends at the state level.

Sexually transmitted infections (STIs) received little attention during the pandemic, but the truth is the STIs did not stop infection increases shortly after the quarantine.

Although medical masks were noninferior to N95 in a multinational study of frontline health care workers, variability in circumstances between countries prevented researchers from drawing firm conclusions.

This unusual Case Study describes a young woman who presented with an acute onset of headaches and dizziness and was found to have aseptic meningitis.