
In 2020, COVID-19 mortality rates were 5 times higher among adults in low socioeconomic positions.

In 2020, COVID-19 mortality rates were 5 times higher among adults in low socioeconomic positions.

The FDA and other health agencies are investigating fresh organic strawberries, branded as FreshKampo or HEB, that are believed to be the source of hepatitis A infections in the US and Canada.
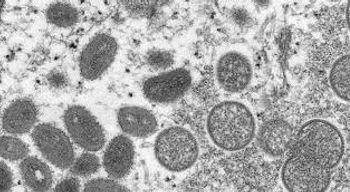
Community transmission of monkeypox outside traditional endemic regions of the virus prompts calls for increased vigilance.

At a media briefing this morning, Infectious Diseases Society of America (IDSA) experts discussed the worldwide 600 confirmed cases of monkeypox.

Synairgen's inhalable Interferon-beta treatment, SNG001, may be a treatment option for hospitalized and immunocompromised COVID-19 patients.
The organization is behind community-based funding and educational programs to propel a healthier society at a grassroots level.

A clinician discusses the disease burden of reoccurring CDI, but sees a silver lining in the new investigational therapies potentially changing the treatment paradigm for the better.
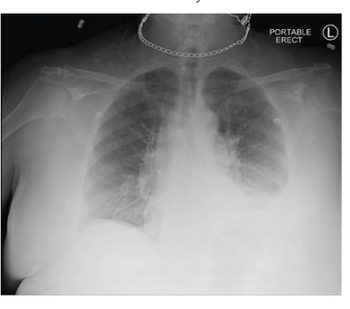
Although this patient had none of these common risk factors associated with Candida empyema, she did have other factors predisposing her to fungal colonization and subsequent infection.
Medical professionals discuss how they affect immunity as well as the necessity of these additional vaccinations.

Four lessons learned from the AIDS crisis that can be applied to the fight against our current pandemic.
Conflicting priorities of infection control, antimicrobial stewardship, and critical care make the management of sepsis secondary to SARS-CoV-2 infection challenging.
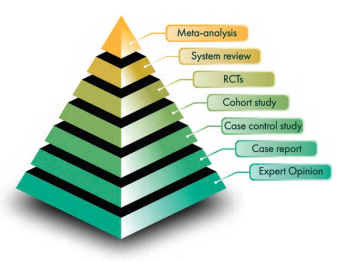
With few exceptions, COVID-19 therapies have not been studied comparatively, so choosing therapies to preferentially recommend is difficult and is done, instead, by strength of evidence and quality of data.

A clinician is advocating for incorporating diagnostic stewardship practices within antimicrobial stewardship programs, which could someday lead to best practice guidelines on a larger scale.
Available literature on thrombosis occurrence in COVID-19 patients focuses on hospitalized cases. A new study sheds light on the incidence of thromboembolism in less severe cases of disease,

Utilizing a newer technology, investigators wanted to see if a reduction in time led to clinically significant antimicrobial adjustments.

A multicenter study looked at treating these infections with omadacycline.

Study links inappropriate, off-guideline antibiotics to increased, avoidable allergic and adverse events in children.
The investigational, fully human IgG1 monoclonal antibody ADG20 prevented and treated infection with COVID-19 variants of concern.

The specialty of the prescribing clinician played a role in the appropriateness of the prescribing for managing ARI in patients with and without HIV.

A medical center wanted to review prescribing and documentation practices for their pharmacists.
There are approximately 300 reported cases of monkeypox in about a dozen countries. However, Dr. Peter Hotez believes we are well equipped to prevent and treat the viral infection.

A new study from the CDC underscores the importance of staying up to date on COVID-19 vaccines and boosters, showing a rapid drop in vaccine effectiveness among children during the Omicron wave that increased with booster doses.

Verrica received a Complete Response Letter from the FDA regarding their New Drug Application for the topical molluscum contagiosum treatment, VP-102.

An expert cautions against panic but advises careful study and robust public health response.
RBX2660, an investigational microbiota-based live biotherapeutic for the treatment of C difficile, safely and effectively reduced recurrent C diff for 6 months.

With limitations on fidaxomicin at their facility, a hospital looked at vancomycin usage to determine if they needed to align with the new IDSA/SHEA CDI treatment guideline.

New information suggests a few more cases have surfaced in the United States, pending CDC confirmation.

A team of investigators studied Clostridioides difficile infection (CDI) isolates to gauge resistance in the 2 antibiotics.

Seres Therapeutics shared phase 3 trial results demonstrating their investigational microbiome-based therapeutic, SER-109, prevented recurrent C difficile infection (rCDI) in 88% of recipients.

Companies say the 3 doses of their vaccine meet all immunobridging criteria required for the Emergency Use Authorization (EUA).