A recent study investigates what causes relapse in those with multiple sclerosis and sought to glean insight into the brain activity of patients with MS during respiratory infections.

A recent study investigates what causes relapse in those with multiple sclerosis and sought to glean insight into the brain activity of patients with MS during respiratory infections.

Negative pressure isolation rooms will be in high-demand during an outbreak of SARS, MERS, or pandemic flu, but how will we meet these needs?
Lucy B. Palmer, MD, from Stony Brook University School of Medicine in New York argues that inhaled antibiotic therapy provides higher drug concentrations of antibiotics with fewer systemic side effects than IV therapy.
Researchers find that performing a TB diagnostic test in a clinic as opposed to a centralized laboratory greatly reduced patients’ time to treatment.
Researchers from the University of Mississippi report a case of an HIV-positive man with pneumonia due to MRSA who was treated with dalbavancin.
The results of a new study reveal increased risk of cardiovascular disease in adults previously hospitalized for pneumonia or sepsis.

New research on Zika, cases of swine flu infections, parallels between Zika and HIV, a new warning sign of HIV infection, and an increase in cyclospora cayetanensis infections in United States make up the top 5 articles this week.

Jason Pogue, PharmD, BCPS-ID, explains how ceftolozane-tazobactam differs from other beta-lactams for treating Pseudomonas infections.
Researchers believe they have developed an effective genetic roadmap to assist in the identification and characterization of infections in the lower respiratory tract.

The Clinton County health commissioner, Pamela Walker Bauer, MPH, RS has confirmed 11 cases of H3N2v flu linked with an Ohio fair.

In a new study published in the journal Vaccine, researchers examine the antibody response to the flu vaccine in pregnant women and their babies.

The latest World Health Organization report points to high MERS transmission risk in hospitals.
After Italy’s parliamentary decision to mandate vaccines, France health officials follow suit, requiring parents to vaccinate their children against 11 common illnesses starting in 2018.

As the IAS Conference on HIV Science wraps up this week, this Public Health Watch report takes a closer look at the challenges that remain when it comes to treating HIV.
A recent WHO news release reports that infant immunization rates continue to fall short of the global immunization target of 90%.

Jason Pogue, PharmD, BCPS-ID, explains his team’s study regarding the susceptibility of Pseudomonas isolates to ceftolozane/tazobactam.

Doctor Without Borders is criticizing countries and government agencies alike for seemingly downplaying the impact of tuberculosis around the world.
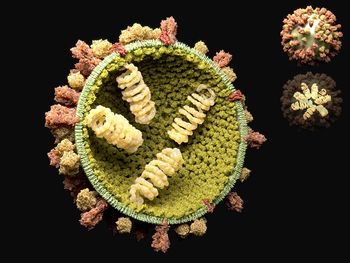
The results of a new study have revealed that that classic “beads on a string” model of the influenza A virus may not be entirely accurate.
A new report from the National Center for Health Statistics has found that too many adults aged 65 and older are missing out on important vaccinations.
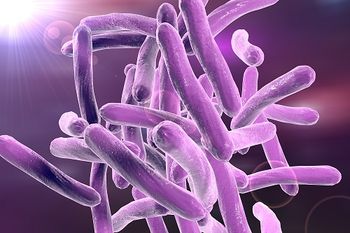
A new study is the first to examine the epidemiology of multidrug-resistant tuberculosis in children in the United States.

A look into how health information exchange interventions are beneficial in HIV care; the discovery of 3 mutations that could help the bird flu spread among humans; news about a new patch formulation for the flu vaccine; progress towards an HIV vaccine; and information on an interactive map that visualizes the US HIV epidemic, make up the Top 5 articles for this week.

Jason Pogue, PharmD, BCPS-ID, explains the limitations of the current drugs for the treatment of Pseudomonas aeruginosa infections.

The number of RSV-infected adults in an African study who also had HIV was quite high, with the burden of HIV disproportionately in the young.

Scientists have identified 3 mutations that could allow the avian influenza strain H7N9 to spread among humans.

Researchers have developed an adhesive patch delivery method for the influenza vaccine and new study shows it is as effective as the flu shot.