HIV / AIDS
Latest News
Latest Videos

CME Content
More News

The specialty of the prescribing clinician played a role in the appropriateness of the prescribing for managing ARI in patients with and without HIV.

The FDA lifted a clinical hold on investigational lenacapavir for HIV treatment and pre-exposure prophylaxis. All clinical studies evaluating injectable lenacapavir can now resume.

Today, the International AIDS Vaccine Initiative (IAVI) and Moderna announced they will soon launch a phase 1 clinical trial for their HIV vaccine candidate, administered with mRNA technology to develop broadly neutralizing antibodies.

The company is utilizing this platform to address new COVID-19 variants, combination vaccines, and potentially prevent HIV.
In Part 2 of a video interview, Sornana Segal-Maurer, MD, discusses the newly published research findings that detail the potential of lenacapavir.
Risk-informed PrEP would reduce HIV incidence by 49 percent over the next 50 years, the study authors found.
Could lenacapavir be the answer for people with multidrug-resistant HIV infection? Principal investigator Sorana Segal-Maurer discusses the promising trial results in this challenging cohort (Interview part 1).

In a study of 32 patients with fourth-line metastatic colorectal cancer, the HIV drug lamivudine slowed disease progression in a quarter of participants.

Children living with HIV had a similar immune response to mRNA COVID-19 vaccination as children without HIV.
Two other male patients were previously known to be in HIV remission, but this is the first woman and first cord blood stem cell transplant case.

HIV reservoirs can remain dormant, reigniting infection at an opportune moment. New strategies seek to reignite these viral reservoirs so they can be identified and destroyed by the immune system.

Correlating immune response to COVID-19 vaccination in persons with HIV to their CD4 T-cell count could inform optimal vaccine strategy.

PrEP prescription initiations and fills dropped during the early parts of the COVID-19 pandemic, according to investigators from the NY State Department of Health.

The meta-analysis included 6 studies, of which 5 were conducted in sub-Saharan Africa, showed that the diagnostic accuracy for Xpert testing was similar to the former symptom screening and rapid test format.

In Germany, HIV pre-exposure prophylaxis users are recommended to receive regular HIV, STI, and renal function testing. How frequently are PrEP users getting tested?

Using CRISPR technology, investigators were able to “knock out” primary human CD4+ T cells, identifying new genes essential to HIV viral replication.

A meta-analysis showed that computerized cognitive training programs produced significant improvements in 6 cognitive and daily function domains among adults living with HIV.

The study authors wrote that selection bias at transmission can shape HIV disease severity and tolerance in different groups.

Today, the FDA approved the abacavir, dolutegravir, and lamivudine single tablet formulation Triumeq, a once-daily HIV treatment for children.

Today, the FDA approved the long-acting HIV treatment Cabenuva (cabotegravir and rilpivirine dually administered) for virologically suppressed adolescents 12 years and older.
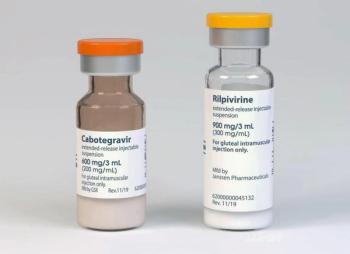
With FDA Approval, the oral lead-in for the cabotegravir and rilpivirine long-acting HIV treatment (Cabenuva) is now optional. Clinical trial data showed similar safety and efficacy profiles for initiating the therapy with or without the oral lead-in.

Congress’s spending bill allocated drastically less funding for HIV testing, prevention, and treatment than previously promised. Carl Schmid, executive director of the HIV+Hepatitis Policy Institute, explains why it is so vital to continue the fight against HIV.

The study authors wrote that few people who initiate tenofovir disoproxil fumarate-based oral PrEP rarely experience clinically significant kidney impairment.

People who use drugs face obstacles, like homelessness and stigma, that hinder their access to healthcare. Dr. Brianna Norton opened the Montefiore-NYHRE Clinic, a first-of its kind academic medical center, to serve marginalized people holistically and respectfully.

The study authors suggest further research and investment to identify areas of support for individuals at the highest risk of poor outcomes.