
A team of investigators from the University of North Carolina Medical Center found that oseltamivir prophylaxis did not reduce the rate of influenza within the first year following a lung transplant.

Increasing Staffing at Long-Term Care Facilities May Reduce Hospitalizations for Pneumonia, Influenza

A team of investigators from the University of North Carolina Medical Center found that oseltamivir prophylaxis did not reduce the rate of influenza within the first year following a lung transplant.

The FDA has approved Mavyret (glecaprevir and pibrentasvir) tablets to treat all 6 genotypes of hepatitis C in children 12 to 17 years.

A 21-week-long flu season begins to wind down while investigators find that flu infections in consecutive seasons are more likely in young children.
The rate of health care-associated influenza cases per season decreased from a median of 16.8 cases pre-policy rollout to 5.2 cases post-policy rollout at a large pediatric hospital.

Could an additive in your food be messing with the immune process that helps you fight off the flu after receiving the influenza vaccine?

Cidara Therapeutics has developed CB-012, a stable conjugate of a highly potent antiviral agent linked to human IgG1 Fc, and investigators are evaluating the asset for the treatment and prevention of seasonal and pandemic influenza A and B infections.
Following years of research and development, NIAID scientists have begun enrolling participants in the first human trial of a universal flu vaccine candidate.

An antiviral drug that could offer months of protection against the flu may be on the horizon and ready to fill in some of the gaps of the seasonal flu vaccine.
The American Academy of Pediatrics is giving its recommendation to the nasal spray form of the influenza vaccine for the 2019-2020 flu season, meaning parents will have 2 choices when getting their children vaccinated this fall.
A recent study has found that a new method of testing circulating H3N2 flu viruses may help flu experts in selecting seasonal vaccine components.

Investigators at Georgia State University have received a federal grant to explore if a microneedle patch could be a universal flu vaccine candidate.

Following a 1-month delay, WHO experts have issued a recommendation for a new influenza A(H3N2) component for next flu season.

A study reports that estimates of influenza epidemic outcomes may be improved by focusing on behavioral discrepancies in different demographics.

Solving a years-old mystery, investigators have discovered that MHC-II molecules can provide a gateway for bat influenza to infect human cells.

Prior studies have indicated that patients with influenza A infections fare worse than those with influenza B, and now a new study backs it up.

Influenza vaccines may be less effective in older adults due to a lack of antibody diversity, a new study suggests.

Although influenza A H1N1 continues to dominate the current flu season in the US, H3N2 viruses are on the rise in some parts of the country.
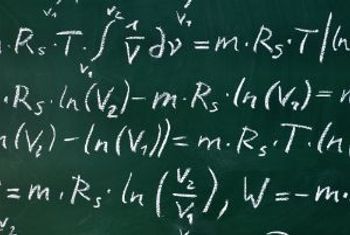
A computer simulation model developed by NYU investigators uses math to forecast influenza activity.
As flu activity remains elevated in all regions of the United States for the fifth week in a row, a new study quantifies the impact of flu vaccination last season.

Japanese researchers find a concerning resistance to a new antiviral flu medication.

Giving an influenza vaccine to hospitalized patients prior to discharge might also be the only opportunity to vaccinate those patients.

Mitsuru Toda, MS, PhD, discusses research that discovered 94 patients who had invasive aspergillosis with influenza.

The FDA has approved the use of the 0.5 mL dose of Fluzone Quadrivalent influenza vaccine to include children age 6 through 35 months.

In a multi-institution collaboration assessing 22 influenza forecasting models, the majority of models consistently showed higher accuracy than historical baseline models.

CDC has released mid-season estimates indicating approximately 84,000 Americans have been hospitalized for the influenza so far this season.