Respiratory Infections
Latest News

Latest Videos

CME Content
More News

New 21-valent conjugate vaccine authorized to combat invasive pneumococcal disease and pneumonia in individuals aged 18 and older.
Kansas now has an outbreak with at least 10 confirmed cases with an expectation from the state's health officials that there will be more.

Heather Platt, MD, discusses Merck’s ongoing research into real-world effectiveness and cost-effectiveness and offers a preview of data to be presented at ESCMID 2025.
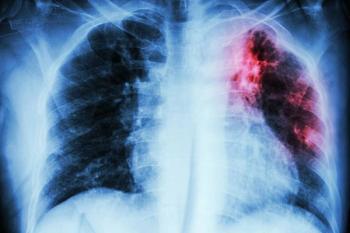
Studies highlight the role of home visits, chest radiography, and sputum monitoring in TB prevention and treatment outcomes.

A 26% rise in childhood tuberculosis cases underscores the need for strengthened global TB control efforts.
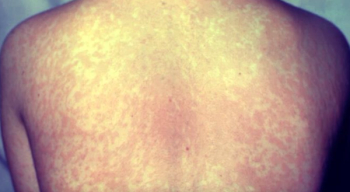
Cases in the US continue to increase, and a new analysis shows 2024 was the highest year for measles cases in Europe in 25 years.

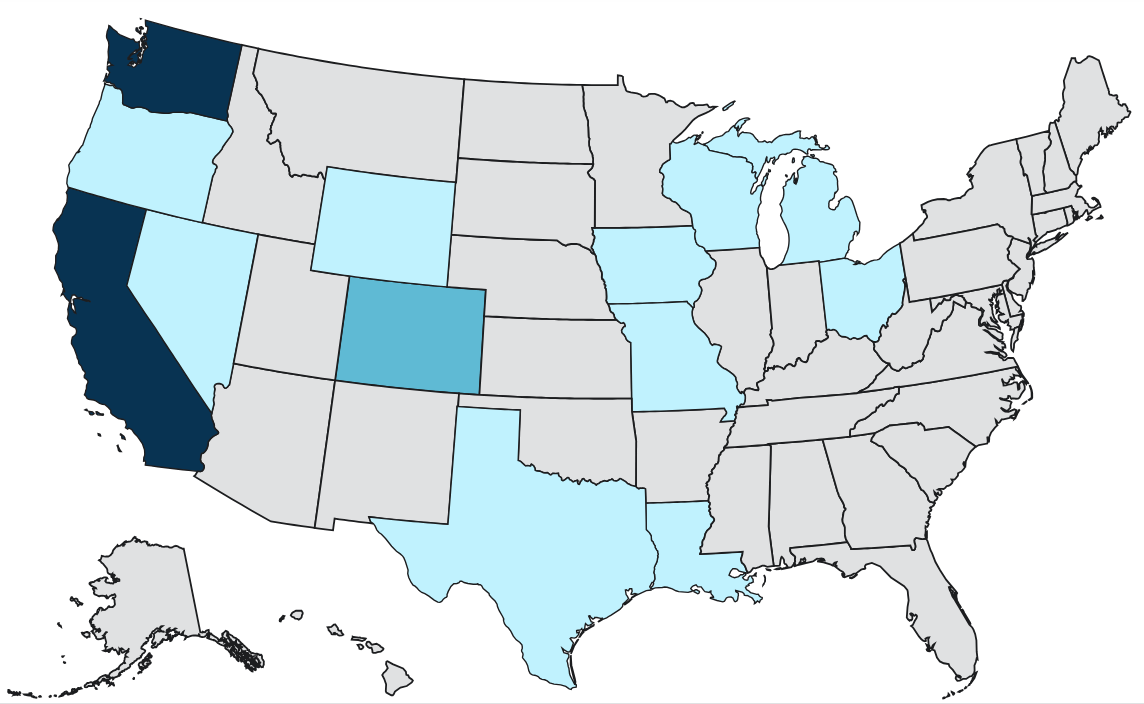
Texas is experiencing its largest measles outbreak in 30 years and the Centers for Disease Control and Prevention's (CDC) Epidemic Intelligence Service is providing support there. Here is an overview on testing, disease presentation, and vaccination, as well as commentary on the public health impact of vaccine hesitancy, and the unintended consequences of the immunization program’s success.

The official cause of death is still under investigation by the New Mexico Office of the Medical Investigator, though the individual tested positive for the measles virus.

Approved vaccines for chikungunya, meningococcal disease, updates on HIV prevention, key regulatory decisions, and more.

New study by Gabriel Nussbaum, MD, PhD, et al, reveals how P gingivalis escapes immune detection, contributing to gum disease and increasing risks for systemic infections such as respiratory disease.
The state’s health department has confirmed all 3 cases are in people who are unvaccinated.
The CDC tracks rising human infections linked to poultry and dairy exposure, while the USDA steps up with a comprehensive strategy to combat HPAI and stabilize egg prices.

Two cats became severely ill, and both owners declined to be tested.

A state official says the woman is hospitalized in another state, and had preexisting health conditions that can make people more vulnerable to illness.

In the Phase 3 trial, GSK's vaccine met all 11 primary endpoints, providing expanded options for preventing invasive meningococcal disease in individuals aged 10-25 years.
Twenty-four cases have been identified within the last two weeks, and 22 of those cases are in children. Nine of the cases have required hospitalization.

Heather Platt, MD, discusses Merck’s latest achievement with Capvaxive and its potential to significantly reduce invasive pneumococcal disease and pneumonia in high-risk adult populations across Europe.
The affected person had a mild case and is recovering.

COVID-19 Accelerates Progression of Atherosclerotic Plaque, Increases Risk for Cardiovascular Events
SARS-CoV-2 infection was associated with rapid growth of plaque in the coronary arteries and an increased risk of cardiovascular events compared to noninfected patients.

Biocontainment, including the most recent threat of highly pathogenic avian influenza, demands we continue to finance research, and continue cooperation with important organizations like the WHO.
The outbreak is currently concentrated in 2 counties in the state, Wyandotte and Johnson.

The COVID-19 pandemic underscored the urgent need for global pandemic preparedness. The World Health Organization (WHO) is leading efforts to establish a global treaty to address future pandemics, with findings expected in 2025. Central to this preparedness is immunomics, a field leveraging advanced genomics technologies to study the immune system at unprecedented levels of detail.
Review finds Long COVID patients experience executive function impairments impacting tasks like shifting, inhibition, and working memory, affecting daily activities.

Robert H. Hopkins, Jr, MD, medical director, National Foundation for Infectious Diseases (NFID) provides an update on incidence rates and virus patterns across different regions of the US.

The federal agency is asking hospitalists and labs to accelerate this process in order to know if they are dealing with avian influenza.