
Children under 5 became infected with the Omicron variant 6-8 times more frequently than young children who contracted Delta. However, Delta COVID-19 infections were more severe than Omicron.

Children under 5 became infected with the Omicron variant 6-8 times more frequently than young children who contracted Delta. However, Delta COVID-19 infections were more severe than Omicron.
Here is a rundown of the most popular stories we covered this past week.

Despite lower comorbidity risk scores than Black or White COVID-19 patients, Mississippi’s Indigenous populations had significantly higher in-hospital mortality rates.
A recent study showed that 25% of mattresses within 4 hospitals required complete replacement, underlying a serious health risk that could lead to unnecessary infections.

The Food and Drug Administration (FDA) said the company is concerned about a limited number of jars that may contain a small fragment of stainless steel from a piece of manufacturing equipment.
The federal agency's new guidelines for adults between 19-59 years replaces risk-based recommendations.
2 doses of the Pfizer-BioNTech vaccine reduced Omicron hospitalizations by 68% in children 5-11 and by 40% in adolescents 12-18 years old.

Surveillance of N meningitidis finds that resistance is rare, with intermediate susceptibility to penicillin consistent year-to-year.

Today, the FDA approved the abacavir, dolutegravir, and lamivudine single tablet formulation Triumeq, a once-daily HIV treatment for children.
The Center for Global Development is launching a new working group, which aims to address antimicrobial resistance by exploring how to improve incentives for pharmaceutical companies to develop new antimicrobials for low-income and middle-income countries (LMICs).

The Pfizer-BioNTech and Moderna mRNA vaccines were found to produce different antibody and killer T-cell responses, suggesting a “mix and match” booster approach may provide the best protection against COVID-19.
“Together these 2 diseases create a perfect storm”: Black people with cancer were more likely to have severe or fatal COVID-19 disease.

New policy appears inconsistent and, on the surface, unfair, expert says.

Today, the FDA approved the long-acting HIV treatment Cabenuva (cabotegravir and rilpivirine dually administered) for virologically suppressed adolescents 12 years and older.

The new action by the federal agency now means the additional booster shot can be administered to people 50 years and older.

The World Health Organization updated its guidelines for the treatment of nonsevere tuberculosis in children after a study showed a four-month duration was noninferior to six months.
The coronavirus pandemic caused the first global recorded decline in human life expectancy.

The Assistant Secretary for Preparedness and Response (ASPR) announced a pause in sotrovimab distribution in regions where BA.2 is the dominant COVID-19 variant, citing evidence that the monoclonal antibody therapy would not effectively neutralize the BA.2 Omicron variant.

The federal agency is expected to provide the EUAs prior to their next VRBPAC meeting on April 6.
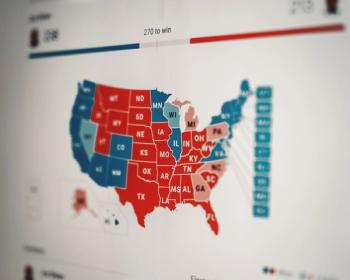
The public health policies enacted in republican and democratic states have never been more different, and the gulf shows no signs of narrowing.

Both noncarbapenem β-lactams and carbapenems produced good outcomes in UTI from β-lactamase-producing Enterobacterales.
Tori Pipak, a physician assistant at Allegheny Health Network’s Positive Health Clinic, details the comprehensive services the clinic provides to people living with HIV.

Check out the important stories we covered this week.

Treating long-lasting nets with a new insecticidal combination that rendered mosquitos unable to fly reduced malaria infections in Tanzanian children by almost half.

People with immune dysfunction are at increased risk for COVID-19 breakthrough infection after vaccination, and should take additional precautions.

Study of Heartland virus in lone star ticks in Georgia adds to evidence of how it evolves, spreads geographically, and causes disease.

The company is developing a single combination vaccine to cover SARS-CoV-2, influenza, and respiratory syncytial virus (RSV) as well as another candidate for endemic human coronaviruses.
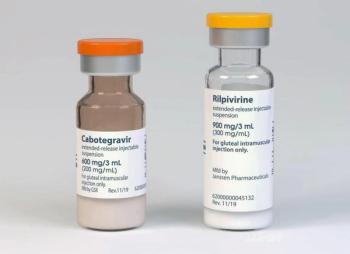
With FDA Approval, the oral lead-in for the cabotegravir and rilpivirine long-acting HIV treatment (Cabenuva) is now optional. Clinical trial data showed similar safety and efficacy profiles for initiating the therapy with or without the oral lead-in.

This designation of the company’s respiratory syncytial virus (RSV) vaccine candidate, PF-06928316, will help to expedite its development and review.

The findings are significant given that a second booster dose may soon be recommended.