
Antibiotics
Latest News
Advertisement
Latest Videos
CME Content
Advertisement
More News








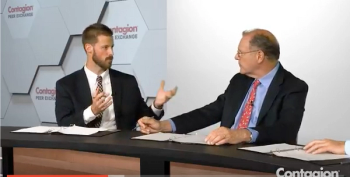





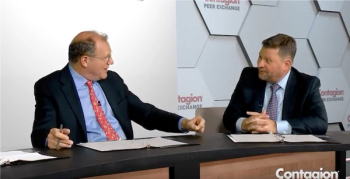


IMI/REL was effective against isolates from the United States and Europe.







Advertisement
Advertisement
Trending on Contagion Live
1
Pritelivir Improves Outcomes in Immunocompromised Patients With Refractory Herpes Simplex Virus
2
Measles Update: February 6, 2026
3
HIV in the Black Community: Shifting from Stigma and Health Inequalities to Communication and Care Initiatives
4
Antibiotics Deconstructed: IV Fosfomycin
5








































































































































